
ETEXT+MASTERINGCHEMISTRY STANDALONE AC
7th Edition
ISBN: 9781269736947
Author: Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 7.10, Problem 23P
Rank the following from strongest base to weakest base:
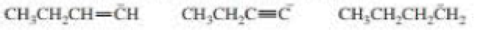
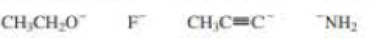
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Rank the following compounds from strongest acid to weakest acid:
Which one is the strongest base?
Rank the given compounds in order of decreasing basicity. 1=most basic, 4=least basic
Chapter 7 Solutions
ETEXT+MASTERINGCHEMISTRY STANDALONE AC
Ch. 7.1 - What is the molecular formula for a monocyclic...Ch. 7.1 - Prob. 2PCh. 7.1 - Draw the structure and give the common and...Ch. 7.1 - Prob. 4PCh. 7.1 - Name the following:Ch. 7.2 - Prob. 6PCh. 7.2 - Name the following:Ch. 7.3 - Prob. 8PCh. 7.3 - Why does cis-2-butene have a higher boiling point...Ch. 7.4 - What orbitals are used to form the carbon-carbon ...
Ch. 7.6 - Prob. 12PCh. 7.6 - Prob. 13PCh. 7.7 - Prob. 14PCh. 7.7 - Which alkyne should be used for the synthesis of...Ch. 7.7 - Prob. 16PCh. 7.8 - Prob. 17PCh. 7.8 - Only one alkyne forms an aldehyde when it...Ch. 7.9 - Describe the alkyne you should start with and the...Ch. 7.9 - Prob. 20PCh. 7.10 - Prob. 21PCh. 7.10 - Prob. 22PCh. 7.10 - Rank the following from strongest base to weakest...Ch. 7.12 - Prob. 26PCh. 7 - What is the major product obtained from the...Ch. 7 - Draw a condensed structure for each of the...Ch. 7 - A student was given the structural formula of...Ch. 7 - Prob. 30PCh. 7 - What is each compounds systematic name?Ch. 7 - What reagents should be used to carry out the...Ch. 7 - Prob. 33PCh. 7 - Prob. 34PCh. 7 - Prob. 35PCh. 7 - Prob. 36PCh. 7 - What is the major product of the reaction of 1 mol...Ch. 7 - What is each compounds systematic name? a....Ch. 7 - What is the molecular formula of a hydrocarbon...Ch. 7 - Answer Problem 39, parts a-b, using 2-butyne as...Ch. 7 - Prob. 41PCh. 7 - a. Starting with 3-methyl 1-butyne, how can you...Ch. 7 - Prob. 43PCh. 7 - Which of the following pairs are keto-enol...Ch. 7 - Prob. 45PCh. 7 - Do the equilibria of the following acid-base...Ch. 7 - What stereoisomers are obtained when 2-butyne...Ch. 7 - Draw the keto tautomer for each of the following:Ch. 7 - Show how each of the following compounds can be...Ch. 7 - A chemist is planning to synthesize 3-octyne by...Ch. 7 - Prob. 51PCh. 7 - What stereoisomers are obtained from the following...Ch. 7 - Prob. 53PCh. 7 - Prob. 54PCh. 7 - Prob. 55PCh. 7 - Prob. 56PCh. 7 - Prob. 57P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Summarize the relationship between pKa and acid strength by completing the following sentences: a. The higher the pKa of an acid, the stronger or weaker the acid. b. The lower the pKa of an acid, the stronger or weaker the acid.arrow_forwardFor each molecule below, draw the conjugate acid or conjugate base or both if the molecule hasboth a conjugate acid and a conjugate base (e.g., water).arrow_forwardComplete the equation for the reaction between each Lewis acid-base pair. In each equation, label which starting material is the Lewis acid and which is the Lewis base; use curved arrows to show the flow of electrons in each reaction. In doing this problem, it is essential that you show valence electrons for all atoms participating in each reaction. (a) (b) (c) (d)arrow_forward
- As we shall see in Chapter 19, hydrogens on a carbon adjacent to a carbonyl group are far more acidic than those not adjacent to a carbonyl group. The anion derived from acetone, for example, is more stable than is the anion derived from ethane. Account for the greater stability of the anion from acetone.arrow_forwardIn each pair, select the stronger acid. (a) Pyruvic acid (pKa 2.49) or lactic acid (pKa 3.08) (b) Citric acid (pKa1 3.08) or phosphoric acid (pKa1 2.10)arrow_forwardRank the following alcohols from strongest to weakest acid:arrow_forward
- Rank the following compounds in their correct order of acidity. 1=Most acidic and 4=least acidic.arrow_forwardA) for each compound show its conjugate base. lone pairs have been left out. B) rank the conjugate base in the order you would predict, from most to least stable. C) rank the original compounds in order, from strongest to weakest acid.arrow_forwardRank the compounds in each of the following groups from strongest acid to weakest acid:arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
General Chemistry | Acids & Bases; Author: Ninja Nerd;https://www.youtube.com/watch?v=AOr_5tbgfQ0;License: Standard YouTube License, CC-BY