
Concept explainers
(a)
Interpretation: The systematic name and common name of the given organic compound is to be interpreted.
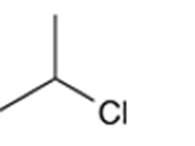
Concept Introduction: Organic compounds are chemical compounds that are mainly composed of carbon and hydrogen atoms. The naming of organic compounds is followed the IUPAC rules. The longest chain of carbon atoms is considered the parent chain. The substituents must be given with the least locant number. The locant number must be written as the prefix in the IUPAC name.
(a)
Answer to Problem 47PP
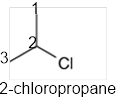
The systematic name of the given compound must be 2-chloropropane and the common name is isopropyl chloride.
Explanation of Solution
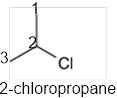
In the given organic compound, the parent longest chain contains 3 carbon atoms so the root word must be propane with one substituent; chloro at C2 position. Thus, the systematic name of the given compound must be 2-chloropropane and the common name is isopropyl chloride.
(b)
Interpretation: The systematic name and common name of the given organic compound is to be interpreted.
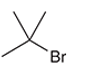
Concept Introduction: Organic compounds are chemical compounds that are mainly composed of carbon and hydrogen atoms. The naming of organic compounds is followed the IUPAC rules. The longest chain of carbon atoms is considered the parent chain. The substituents must be given with the least locant number. The locant number must be written as the prefix in the IUPAC name.
(b)
Answer to Problem 47PP
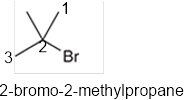
The systematic name of the given compound must be 2-bromo-2-methylpropane and the common name is tert-butyl bromide.
Explanation of Solution
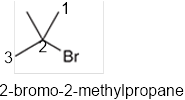
In the given organic compound, the parent longest chain contains 3 carbon atoms so the root word must be propane with two substituents: chloro and methyl at 2 positions. Thus, the systematic name of the given compound must be 2-bromo-2-methylpropane and the common name is tert-butyl bromide.
(c)
Interpretation: The systematic name and common name of the given organic compound is to be interpreted.

Concept Introduction: Organic compounds are chemical compounds that are mainly composed of carbon and hydrogen atoms. The naming of organic compounds is followed the IUPAC rules. The longest chain of carbon atoms is considered the parent chain. The substituents must be given with the least locant number. The locant number must be written as the prefix in the IUPAC name.
(c)
Answer to Problem 47PP
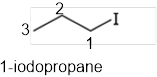
The systematic name of the given compound must be 1-iodopropane and the common name is propyl iodide.
Explanation of Solution
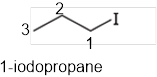
In the given organic compound the parent longest chain contains 3 carbon atoms so the root word must be propane with one substituent; iodo at 1 position. Thus, the systematic name of the given compound must be 1-iodopropane and the common name is propyl iodide.
(d)
Interpretation: The systematic name and common name of the given organic compound is to be interpreted.
Concept Introduction: Organic compounds are chemical compounds that are mainly composed of carbon and hydrogen atoms. The naming of organic compounds is followed the IUPAC rules. The longest chain of carbon atoms is considered the parent chain. The substituents must be given with the least locant number. The locant number must be written as the prefix in the IUPAC name.
(d)
Answer to Problem 47PP
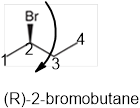
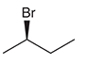
The systematic name of the given compound must be (R)-2-bromobutane and the common name is (R)- sec-butyl bromide.
Explanation of Solution
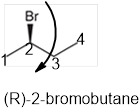
In the given organic compound the parent longest chain contains 4 carbon atoms so the root word must be butane with one substituent; bromo at C2 position. Thus, the systematic name of the given compound must be (R)-2-bromobutane and the common name is (R)- sec-butyl bromide. The prefix (R) indicates the clockwise rotation of the groups bonded at the chiral C atom when arranged from 1 to 4 in the increasing order of their
(e)
Interpretation: The systematic name and common name of the given organic compound is to be interpreted.
Concept Introduction: Organic compounds are chemical compounds that are mainly composed of carbon and hydrogen atoms. The naming of organic compounds is followed the IUPAC rules. The longest chain of carbon atoms is considered the parent chain. The substituents must be given with the least locant number. The locant number must be written as the prefix in the IUPAC name.
(e)
Answer to Problem 47PP
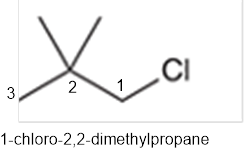
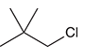
The systematic name of the given compound must be 1-chloro-2,2- dimethylpropane and common name is neopentyl chloride.
Explanation of Solution
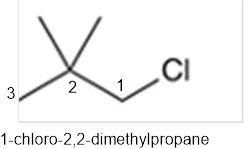
In the given organic compound, the parent longest chain contains 3 carbon atoms so the root word must be propane with three substituents as methyl and chloro at C1 and C2 position. Thus, the systematic name of the given compound must be 1-chloro-2,2- dimethylpropane and the common name is neopentyl chloride.
(f)
Interpretation: The systematic name and common name of the given organic compound is to be interpreted.
Concept Introduction: Organic compounds are chemical compounds that are mainly composed of carbon and hydrogen atoms. The naming of organic compounds is followed the IUPAC rules. The longest chain of carbon atoms is considered the parent chain. The substituents must be given with the least locant number. The locant number must be written as the prefix in the IUPAC name.
(f)
Answer to Problem 47PP
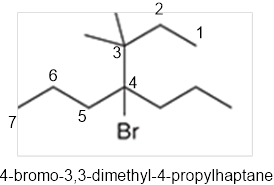
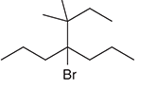
The systematic name of the given compound must be 4-bromo-3,3-dimethyl-4-propylhaptane.
Explanation of Solution
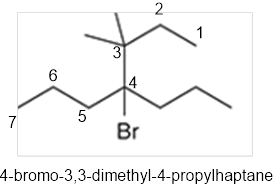
In the given organic compound the parent longest chain contains 7 carbon atoms so the root word must be haptane with four substituents as propyl, methyl and bromo. Thus, the systematic name of the given compound must be 4-bromo-3,3-dimethyl-4-propylhaptane.
Want to see more full solutions like this?
Chapter 7 Solutions
EBK ORGANIC CHEMISTRY, ENHANCED ETEXT
- Draw the structures for the following compounds.(a) 3-benzyl-4-bromohexane , 4,4-dimethylcyclohexene(b) trans-4,5-dibromohex-2-ene , cis-1,1-dibromo-2-ethyl-2,3-dimethylcyclobutanearrow_forwardArrange the following compounds in increasing order of their property as indicated :(i) CH3COCH3, C6H5COCH3, CH3CHO(reactivity towards nucleophilic addition reaction)(ii) Cl—CH2—COOH, F—CH2—COOH, CH3—COOH (acidic character)arrow_forwardDraw as many compounds as you can that fit the following descriptions:arrow_forward
- Draw the structure of each of the following molecules. (a) hexanedinitrile; (b) (S)-4-nitroheptanenitrile; (c) 4,4-diethylcyclohexanecarbonitrilearrow_forwardCompound B contains one chiral carbon atom and reacts with concentrated H2SO4 to yield compounds and G (both CSH10) that are positional isomers of each other (neither compound is capable of having geometric isomers). Provide the structures of these compounds in the boxes below. No IUPAC names are necessary. Farrow_forwardDraw structures that t each description and name the functional group in each molecule: (a) two constitutional isomers with molecular formula C5H10O that contain different functional groups; (b) two constitutional isomers with molecular formula C6H10O that contain the same functional group.arrow_forward
- Give the molecular, structural and displayed formulae of the following molecules: (a) 2,2-dimethylpropane (b) 3-bromo-2, 4, 4-trimethyloctanearrow_forwardDraw the structural formulas for the following compounds. Include all the bonds to hydrogen atoms. Be sure to answer both parts. (a) 1,2,4-trimethylbenzene: (b) chlorobenzene:arrow_forward(b) Draw the structural formula for each of the following compounds. Lukiskan formula struktur bagi setiap sebatian berikut. (i) 3-fluoro-2,4,4-trimethylpent-2-ene (ii) ethyl butanoate (ii) 2,4-dichloro-3-methylpentanoic acidarrow_forward
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning



