
Concept explainers
(a)
Interpretation:
The energy difference between the two chair conformations of
Concept introduction:
The conformation of cyclohexane is a type of
Answer to Problem 7.14P
The energy difference between the two chair conformations of
Explanation of Solution
The two chair conformations of
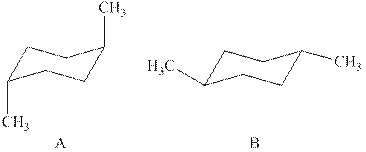
Figure 1
The
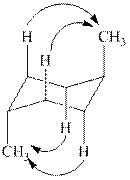
Figure 2
Thus, there are total four
The given value of contribution one
In case of chair conformation B, there is no
Therefore, the energy difference of between the two chair conformations of
Substitute the values of energies in above equation.
Thus, the energy difference is
The energy difference between the two chair conformations of the given compound is
(b)
Interpretation:
The energy difference between
Concept introduction:
The conformation of cyclohexane is a type of
Answer to Problem 7.14P
The energy difference between
Explanation of Solution
The chair conformations of
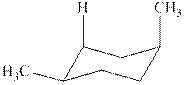
Figure 3
The
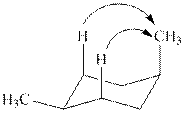
Figure 4
The given value of contribution one
The most stable chair conformation of
![]()
Figure 5
In case of most stable chair conformation of
Therefore, the energy difference of between the two chair conformations of
Substitute the values of energies in above equation.
Thus, the energy difference is
The energy difference between
Want to see more full solutions like this?
Chapter 7 Solutions
ORGANIC CHEMISTRY (LL)+ SAPLING ACC >BI
- Draw Newman's projections along the C3¬C4 bond to show the most stable and least stable conformations of 3-ethyl-2,4,4-trimethylheptane.arrow_forwardDraw (1R,2S,3R)-1bromo-3-fluoro-2-propyl cyclohexane in 2D formarrow_forwardDraw the most stable conformation of pentane, using wedges and dashes to represent bonds coming out of the paper and going behind the paper, respectively.arrow_forward
- Draw the most stable conformation of 1,4-dichlorobutane, using wedges and dashes to represent bonds coming out of the paper and going behind the paper, respectively.arrow_forwardDraw and name the seven aldehydes and ketones with the formula C5H10O. Which are chiral?arrow_forwardA 1, 2-cis disubstituted cyclohexane, such as cis-1, 2-dichlorocyclohexane, must have one group axial and one group equatorial. Explain.arrow_forward
- Draw structures that meet the following descriptions (there are many possibilities): (a) Three isomers with the formula C8H18 (b) Two isomers with the formula C4H8O2arrow_forwardConsider 1-bromo-2-methylpropane and draw the following. (a) The staggered conformation(s) of lowest energy (b) The staggered conformation(s) of highest energyarrow_forwardWhat is the stereochemical configuration of the enantiomer of (2S, 4R)-2, 4-octanediol? (A diol is a compound with two —OH groups.)arrow_forward
- Consider 1-bromopropane, CH3CH2CH2Br. (a) Which of these is the lowest energy conformation?arrow_forwardCalculate the difference in Gibbs free energy in kilojoules per mole between the alternative chair conformations of: Q.)cis-4-Methylcyclohexanolarrow_forwardDefine cyclohexane chair conformation ?arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning


