
Concept explainers
Interpretation:
A model of chair cyclohexane corresponding to the leftmost model in the given figure is to be drawn. The two reasons corresponding to the fact that the half-chair conformation is less stable than the chair or twist-boat conformation is to be stated.
Concept introduction:
The conformation of cyclohexane is a type of
Answer to Problem 7.1P
A model of chair cyclohexane corresponding to the leftmost model in the given figure is shown below.
The half-chair conformation is less stable than the chair or twist-boat conformation because half-chair conformation has maximum ring strain and the presence of angle strain in half-chair conformation of cyclohexane molecule.
Explanation of Solution
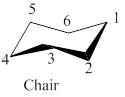
The given leftmost figure of chair conformation of cyclohexane is shown as,
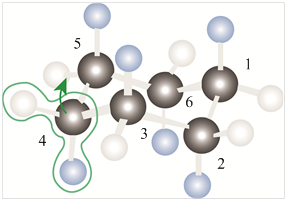
Figure 1
The model of chair conformation of cyclohexane corresponding to the leftmost model is shown as,
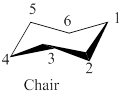
Figure 2
If the carbon-4 in order to locate carbons 2-5 in a common plane then the above model chair conformation of cyclohexane is shown as,
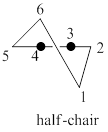
Figure 3
Thus, the raising of carbon-4 in order to locate carbons 2-5 in a common plane forms the half-chair conformation of cyclohexane molecule. This half-chair conformation of cyclohexane molecule is a transition state for the conversion into the chair and twist boat conformations.
The two reasons which explain that the half-chair conformation is less stable than the chair or twist-boat conformation are as follows.
• The ring strain in half chair conformation is more as compared to the chair or twist-boat conformation.
• The angle strain of
A model of chair cyclohexane corresponding to the leftmost model in the given figure has been shown above. The two reasons corresponding to the fact that the half-chair conformation is less stable than the chair or twist-boat conformations has been stated above.
Want to see more full solutions like this?
Chapter 7 Solutions
ORGANIC CHEMISTRY (LL)+ SAPLING ACC >BI
- Urgent explain in details In all 12 structures, highlight the main steric interactions that could be contributing to the energy difference between conformersarrow_forwardBuild a model of methylcyclohexane, and use the model to complete the following Newmanprojections of methylcyclohexane in the chair conformation: a. When the methyl group is in an axial or equatorial (circle one) position, the molecule is inits lowest potential energy conformation. b. Label one Newman projection above anti and the other gauche to describe the relationshipbetween the methyl group and C3 of the ring. c. In general, which is a lower PE conformation, anti or gauche? d. Explain how your answer to b and c provide an explanation for why it is more favorable fora large group to be in an equatorial than an axial position.arrow_forwardFor the molecule given, prioritize each substituent on the stereogenic carbon, and assign absolute stereochemistry.arrow_forward
- Although benzene is normally written with three double bonds, benzene is not reactive towards many reagents that alkenes normally react with. This lack of reactivity can be explained by the unusual stability created by cyclic conjugation. (i) describe at least one of the physical properties of benzene that demonstrates how the true structure of benzene does match the way the structure is normally written (ii) explain how the unusual stability of benzenecan demonstrated by its thermodynamic properties through some form of experiment. (iii) include an appropriately labeled diagram as part of your answer (you do not have to quote any numerical values)arrow_forwardHow the relative stability of the two conformations of any disubstituted cyclohexane can be analyzed ?arrow_forwardMake a model of cyclooctatetraene in the tub conformationarrow_forward
- Explain the concept of Hydrogenation Data and Degrees of Unsaturation ?arrow_forwardName attached diene and state whether the ball-and-stick model shows thediene in the s-cis or s-trans conformation.arrow_forwardDoes eugenol have any double bonds that would be considered E or Z? Explain your answer.arrow_forward
- Explain and give examples of the stability order of carbocations within the group and among different groupsarrow_forwardFor each of the statements in Column I, choose a substituent from Column II that fits the description for the compound on the right:arrow_forwardI am slightly confused on the naming of ethers. How come for chloromethoxymethane, it is not methoxychloride? Isn't chloride more complex than the alkyl group, so it should be placed at the root? Or is it not placed at the root because chloride is not an alkyl group at all?arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

