
Organic Chemistry - Standalone book
10th Edition
ISBN: 9780073511214
Author: Francis A Carey Dr., Robert M. Giuliano
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 7.13, Problem 20P
The
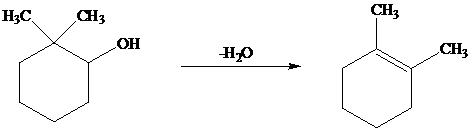
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Reaction of N,N-diethyl-p-diaminobenzene with sodium nitrite and hydrochloric acid at 0°C and subsequent reaction with nitrobenzene.
Reaction of N,N-diethyl-p-diaminobenzene with sodium nitrite and hydrochloric acid at 0°C, followed by treatment with nitrobenzene.
A racemic mixture of 2-methyl-1-phenyl-1-butanone is formed when (R)-2-methyl-1-phenyl-1-butanone is dissolved in an acidic or basic aqueoussolution. Give an example of another ketone that undergoes acid- or base-catalyzed racemization.
Chapter 7 Solutions
Organic Chemistry - Standalone book
Ch. 7.1 - Name each of the following using IUPAC...Ch. 7.1 - Prob. 2PCh. 7.2 - How many carbon atoms are sp2-hybridized in the...Ch. 7.3 - Prob. 4PCh. 7.3 - Are cis-2-hexene and trans-3-hexene stereoisomers?...Ch. 7.4 - Prob. 6PCh. 7.4 - Prob. 7PCh. 7.4 - Give the IUPAC name of each of the compounds in...Ch. 7.5 - Arrange the following in order of increasing...Ch. 7.6 - Prob. 10P
Ch. 7.6 - Standard enthalpies of formation are known for all...Ch. 7.6 - Prob. 12PCh. 7.6 - Despite numerous attempts, the alkene...Ch. 7.6 - Write structural formulas for the six isomeric...Ch. 7.7 - Place a double bond in the carbon skeleton shown...Ch. 7.9 - Identify the alkene obtained on dehydration of...Ch. 7.10 - Prob. 17PCh. 7.11 - Prob. 18PCh. 7.12 - Prob. 19PCh. 7.13 - The alkene mixture obtained on dehydration of...Ch. 7.14 - Write the structures of all the alkenes that can...Ch. 7.14 - Write structural formulas for all the alkenes that...Ch. 7.15 - A study of the hydrolysis behavior of...Ch. 7.15 - Use curved arrows to illustrate the electron flow...Ch. 7.15 - Predict the major product of the reaction shown.Ch. 7.16 - Prob. 26PCh. 7.17 - Prob. 27PCh. 7.18 - Prob. 28PCh. 7.19 - Predict the major organic product of each of the...Ch. 7.19 - A standard method for the synthesis of ethers is...Ch. 7 - Write structural formulas for each of the...Ch. 7 - Prob. 32PCh. 7 - Give an IUPAC name for each of the following...Ch. 7 - A hydrocarbon isolated from fish oil and from...Ch. 7 - Prob. 35PCh. 7 - Prob. 36PCh. 7 - Prob. 37PCh. 7 - Prob. 38PCh. 7 - Choose the more stable alkene in each of the...Ch. 7 - Suggest an explanation for the fact that...Ch. 7 - Prob. 41PCh. 7 - Write structural formulas for all the alkene...Ch. 7 - Prob. 43PCh. 7 - Prob. 44PCh. 7 - Predict the major organic product of each of the...Ch. 7 - Prob. 46PCh. 7 - Prob. 47PCh. 7 - The rate of the reaction In the first order in...Ch. 7 - Prob. 49PCh. 7 - Prob. 50PCh. 7 - You have available 2,2-dimethylcyclopentanol (A)...Ch. 7 - Prob. 52PCh. 7 - Prob. 53PCh. 7 - Prob. 54PCh. 7 - Acid-catalyzed dehydration of...Ch. 7 - The ratio of elimination to substitution is...Ch. 7 - Prob. 57PCh. 7 - Prob. 58DSPCh. 7 - Prob. 59DSPCh. 7 - Prob. 60DSPCh. 7 - Prob. 61DSPCh. 7 - A Mechanistic Preview of Addition Reactions The...Ch. 7 - Prob. 63DSPCh. 7 - Prob. 64DSPCh. 7 - Prob. 65DSP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- When warmed in dilute sulfuric acid, 1-phenyl-1,2-propanediol undergoes dehydration and rearrangement to give 2-phenylpropanal. (a) Propose a mechanism for this example of a pinacol rearrangement (Section 10.7). (b) Account for the fact that 2-phenylpropanal is formed rather than its constitutional isomer, 1-phenyl-1-propanone.arrow_forwardElimination of HBr from 2-bromonorbornane gives only 2-norbornene and no 1-norbornene. How do you account for the regioselectivity of this dehydrohalogenation? In answering this question, you will find it helpful to look at molecular models of both 1-norbornene and 2-norbornene and analyze the strain in each.arrow_forwardWhen 5-bromo-1-pentanol is treated with sodium hydride in diethyl ether, the product is analyzed to be C5H10O. Propose a likely structure for this product, suggesting a reasonable mechanistic pathway for its formationarrow_forward
- we know that ethers, such as diethyl ether and tetrahydrofuran, are quite resistant to the action of dilute acids and require hot concentrated HI or HBr for cleavage. However, acetals in which two ether groups are linked to the same carbon undergo hydrolysis readily, even in dilute aqueous acid. How do you account for this marked difference in chemical reactivity toward dilute aqueous acid between ethers and acetals?arrow_forward5. 2. When 2,2-dimethyl-1-propanol is heated with acid, it is slowly converted into an 85:15 mixture of 2 alkenes of molecular formula C2H10.a) Give the mechanisms by which they are formed. b) Give the structures of these alkenes. c) Identify with reason which would be the major productarrow_forwardGive the substitution and elimination products you would expect from the followingreactions.(a) 3-bromo-3-ethylpentane heated in methanolarrow_forward
- Infer how 2,3-dimethylbutane-2,3-diol yields 3,3-dimethylbutan-2- one in the presence of dehydrating acid, HCl, using mechanistic explanationarrow_forwardAn aldehyde reacts with 1 eqivalent of methanol in the presence of acid to give what expected product? hemiacetal acetal gem-diol racemic mixturearrow_forward1.Describe the ozonolysis of alkenes 2.one mole of a hydrocarbon(A) reacts with one mole of beomine giving a dibromo compound C5H10Br2.Substance A on treatment with cold dilute kMnO4 solution forms a compound C5C12O2(C5H12O2) on ozonolysis A,gives equimolar quantities of propanone and ethanol.Deduce the structure of substance A.arrow_forward
- Provide the reagents necessary to convert (R)-2-chlorobutane to (S)-2-methoxybutane.arrow_forward-Hydroxyketones and -hydroxyaldehydes are also oxidized by treatment with periodic acid. It is not the -hydroxyketone or aldehyde, however, that undergoes reaction with periodic acid, but the hydrate formed by addition of water to the carbonyl group of the -hydroxyketone or aldehyde. Write a mechanism for the oxidation of this -hydroxyaldehyde by HIO4.arrow_forwardPropose a mechanism to account for the formation of a cyclic acetal from 4-hydroxypentanal and one equivalent of methanol. If the carbonyl oxygen of 4-hydroxypentanal is enriched with oxygen-18, do you predict that the oxygen label appears in the cyclic acetal or in the water?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Coenzymes and cofactors; Author: CH15 SWAYAM Prabha IIT Madras;https://www.youtube.com/watch?v=bubY2Nm7hVM;License: Standard YouTube License, CC-BY
Aromaticity and Huckel's Rule; Author: Professor Dave Explains;https://www.youtube.com/watch?v=7-BguH4_WBQ;License: Standard Youtube License