
(a)
Interpretation: The value of
Concept introduction:Thermodynamics is a study of energy transfers that can be done by either heat or work. The energy transferred through work involves force. When work is positive then system gains energy while when work is negative then system loses energy. Heat is not a state function and therefore change in enthalpy of reaction
(b)
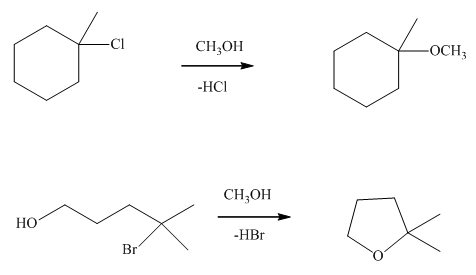
Interpretation: The mechanism using curved arrows should be indicated for below reactions.
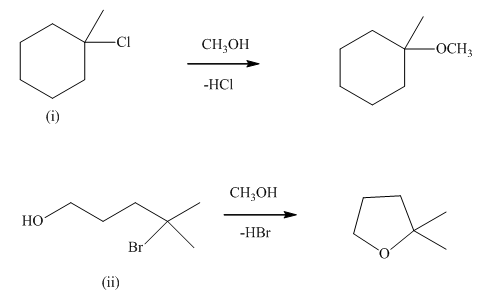
Concept introduction:Unimolecuar substitution or
A general
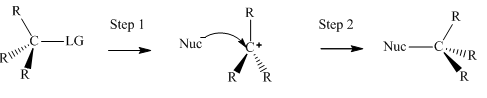
(c)
Interpretation: The effect observed on the rate of methanolysis when the concentration of tertiary
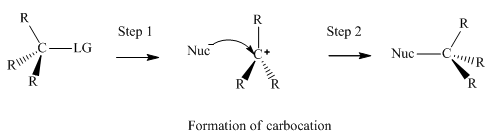
Concept introduction:Unimolecuar substitution or
A general
Want to see the full answer?
Check out a sample textbook solution
Chapter 7 Solutions
Organic Chemistry: Structure and Function
- Explain why the hydrolysis of [Co(NH3)5Cl]2+ in the presence of base muchfaster than that of [Co(py)5Cl]2+? Illustrate your answer with suitable diagrams.arrow_forwardCan you please check my work on the following ochem reaction scheme and let me know if it is correct or what is wrong... the question was: Consider 3,4-dimethylpiperidine being subjected to the following: Step 1: CH3I (excess); Step 2: NaOH, heat Step 3: CH3I (excess); Step 4: NaOH, heat Provide the bond line structures for the major organic product obtained in each step and discuss the regiochemistry for Step 2.arrow_forwardQuestion: How do quantum mechanical effects influence the stability and reactivity of molecules with non-classical carbocations, such as the 2-norbornyl cation, and how does this impact the reaction mechanisms and outcomes?arrow_forward
- Draw the structure of the predominant form (principal species) of 1,3-dihydroxybenzene at pH 9.00 and at pH 11.00. What is the second most prominent species at each pH?arrow_forwardWrite the reaction of 1-bromopentane with sodium ethoxide and show all possible products (E2 and SN2) that could occur. Please don't give handwritten earrow_forwardProvide a full, arrow-pushing mechanism for the reaction of 1-methylcyclohexene with Br2 in water.arrow_forward
- Carbons 1 and 4 of 1,3-cyclopentadiene are equivalent and give the same carbocation on protonation. Likewise, carbons 2 and 3 are equivalent. Write the structure of the carbocation formed by protonation of C-2 or C-3 to verify that it is not allylic and therefore not as stable as the one formed by protonation of C-1 or C-4.arrow_forwardRead these directions carefully. For the reaction of 3-methyl-1-propene with Cl2 and H2O shown below, fill in the details of the mechanism. Draw the appropriate chemical structures and use an arrow to show how pairs of electrons are moved to make and break bonds during the reaction. Be sure to write all lone pairs of electrons and all formal charges. Finally, in the boxes provided by the arrows, write which kind of mechanistic element is being indicated, such as "make a bond", "add a proton", etc.arrow_forwardWhen Br2 is added to buta-1,3-diene at -15 °C, the product mixture contains 60% ofproduct A and 40% of product B. When the same reaction takes place at 60 °C, theproduct ratio is 10% A and 90% B.If you had a solution of pure A, and its temperature were raised to 60 °C, what wouldyou expect to happen? Propose a mechanism to support your prediction.arrow_forward
- Define the Mechanism- Addition of H and BH2—Hydroboration ?arrow_forwardAnilinium ion, PhNH3+ , Catalyze semicarbazone formation from benzaldhyde much more effectively than would be expected on the basis of its strength as an acid. Explain.arrow_forwardIn two parts, outline the Electrophilic aromatic substitution for the nitration of benzene using nitric acid and sulfuric acid: A) In part I, using the curved arrow formalism, outline a mechanism for the formation of the nitronium ion, NO2+ starting from nitric acid, HNO3 and sulfuric acid, H2SO4. Do not forget to show all of the formal charges for each atom that is not zero in this mechanism. B) Starting from the nitronium ion that you generated in part “a” from above, outline the Electrophilic Aromatic Substitution of this ion with benzene.arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

