
(a)
Interpretation: The expected product for the reactions is to be drawn and stereochemistry along with preferred substitution mechanistic pathway between
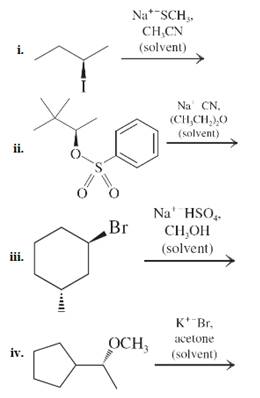
Concept introduction: Unimolecuar substitution or
The general reaction mechanism for the
Step 1: Formation of a carbocation.
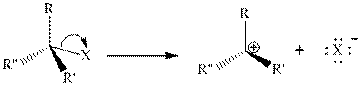
Step 2: Attack of a nucleophile on electron-deficient carbon of a carbocation.
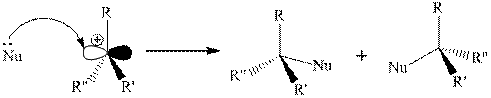
(b)
Interpretation: Whether treatment of
Concept introduction: It has been found experimentally that if the leaving group lies on tertiary carbon than reaction proceeds preferentially via
If the leaving group lies on secondary than both
A good nucleophile can take part in both
Want to see the full answer?
Check out a sample textbook solution
Chapter 7 Solutions
Organic Chemistry: Structure and Function
- A difficult problem in the synthesis of PGF2α is the introduction of the OH group at C15 in the desired configuration. a. Label this stereogenic center as R or S. b. A well known synthesis of PGF2α involves reaction of A with Zn(BH4)2, a metal hydride reagent similar in reactivity to NaBH4, to form two isomeric products, B and C. Draw their structures and indicate their stereochemical relationship. c. Suggest a reagent to convert A to the single stereoisomer X.arrow_forwardStrong support for the mechanism of the nucleophilic aromatic substitutionreaction that proceeds through a benzyne intermediate comes from the reaction shown here, in which bromobenzene is treated with KNH2 in the presence of cyclopentadiene. A product that is isolated has the formula C11H10. Draw the structure of that product and explain how it validates the production of a benzyne intermediate.arrow_forwardConsider the following statement in reference to SN1, SN2, E1, and E2 reactions of haloalkanes. To which mechanism(s), if any, does the statement apply? Involves a carbocation intermediatearrow_forward
- An aldol condensation reaction using the reagents acetone and benzaldehyde, in the presence of sodium hydroxide was used to produce dibenzylacetone. Give a detailed NMR analysis for the product (address the issue of double bond stereochemistry; 3J values are sensitive to double bond geometry see diagram below).arrow_forwardGive steucture of organic and inorganic products of the following sn2 reaction, and identify the nucleophile, substrate, and leaving group.arrow_forwardThe reaction proceeds by an SN2 mechanism. Draw a sketch of the structure of the transitionstate, clearly indicating:- the geometry at the electrophilic carbon centre, and- bonds in the process of being broken or formed with dotted lines, if present.arrow_forward
- Arrange the structure on the image with regard to the reactivity towards nucleophilic acyl substitution 1 being the least and 3 being the mostarrow_forwardWhat is mechanism of ether cleavage Is this cleavage following SN1 or SN2 ? Give your explanation.arrow_forwardWhen 1,2-cyclohexanediol is dehydrated in the presence of concentrated sulfuric acid, the major product is not an alkene. Instead, you get cyclohexanone. Write a reasonable and detailed mechanism for the dehydration of 1,2-cyclohexanediol to form cyclohexanone. Use curved arrows to show the flow of electrons and draw the structures of all intermediates and byproducts formed in the course of this reaction as well as any alternative resonance structures that will help you to account for the formation of the major product observed in this reaction.arrow_forward
- Understanding different substitution and elimination pathways gives useful insight into chemical mechanisms and pathways. Give the different important aspects of the Sn1, Sn2, E1, E2, and E2cB reactions.arrow_forward(a) What reagents would be used for the conversion of alkene A into the target? (b) What reaction is involved in the conversion of alcohol B into alkene A? Suggest a reagent that might affect this transformation. (c) Give a retrosynthetic analysis showing the disconnection of B, the synthons produced that lead to the synthetic equivalents given (draw their structures).arrow_forward(19, 8, 2) Specify a synthetic scheme that would produce the compound shown above in the fewest steps possible. Use one of the starting materials shown together with any of the available reagents. Give the number of the starting material followed by the letters of the reagents in the order of their use, for example: 3be.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





