
Concept explainers
(a)
Interpretation:
The complete, detailed mechanism and products for the given reaction are to be drawn assuming that it takes place via an
Concept introduction:
Answer to Problem 8.13P
The complete, detailed mechanism for the given reaction, assuming that it takes place via an
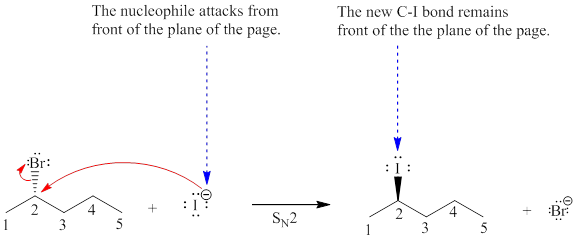
Explanation of Solution
The given reaction is:
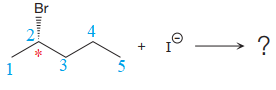
The reactant molecule contains one chiral center at the C2 carbon atom. The leaving group is

In given alkyl halide C2 carbon atom is chiral in nature and affected throughout the course of the reaction. The nucleophile
For the given
(b)
Interpretation:
The complete, detailed mechanism and products for the given reaction are to be drawn assuming that it takes place via an
Concept introduction:
Answer to Problem 8.13P
The complete, detailed mechanism for the given reaction, assuming that it takes place via an
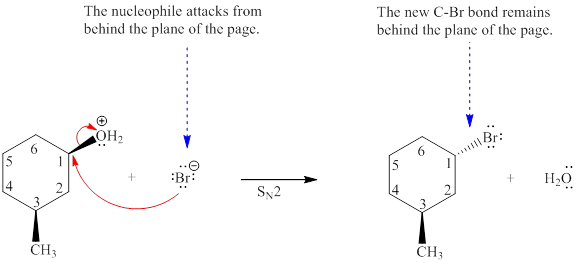
Explanation of Solution
The given reaction is
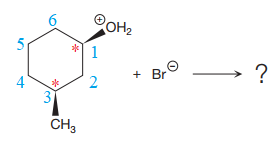
The reactant molecule contains two chiral centers at C1 and C3 carbon atoms. The leaving group is

Note that the stereochemical configuration at the chiral center at C3 remains unchanged because no bonds to it were broken or formed. However, the stereochemical configuration at the chiral center at C1 changes as bonds to it are affected throughout the course of reaction. This will change the stereochemical configuration at C3 chiral carbon atom. Thus, a single stereoisomer will be generated.
For the given
(c)
Interpretation:
The complete, detailed mechanism and products for the given reaction are to be drawn assuming that it takes place via an
Concept introduction:
Answer to Problem 8.13P
The complete, detailed mechanism for the given reaction, assuming that it takes place via an
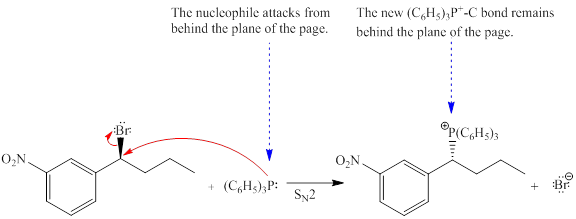
Explanation of Solution
The given reaction is
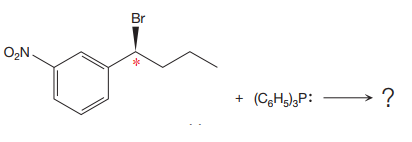
The reactant molecule contains one chiral center. The leaving group is

Note that, the stereochemical configuration at the chiral center changes as bonds to it ae affected throughout the course of reaction. This will change the stereochemical configuration at the chiral center, and it will be opposite than the reactant molecule. Thus, a single stereoisomer will be generated.
For the given
Want to see more full solutions like this?
Chapter 8 Solutions
ORGANIC CHEMISTRY E-BOOK W/SMARTWORK5
- Draw a free-radical mechanism for the following reaction, forming the major monobromination product:arrow_forwardDraw the complete mechanisms leading to the two major products and determine whether the product mixture will be optically active for each of the following reactions.arrow_forwardDraw the mechanism and product with correct stereochemistry for the following reactionarrow_forward
- Please draw the resulting product after each synthetic step and do not show mechanism. Each reaction has at a least two stepsarrow_forwardPlease draw the mechanism of the reaction shown below.arrow_forwarddraw out mechanism (either E1 or E2) for this combined with CH3OH (arrows, intermediates, etc should be shown). Circle the less prevalent and more prevalent productsarrow_forward
- Draw the major product and reaction mechanism for each reaction. Explain why you took the pathway that you did, and how you got the final products.arrow_forwardwhat is the minor product of the following reaction.Please draw the mechanismarrow_forwardPlease draw the major product(s)for the following reactionarrow_forward
- The following reaction is done with a low concentration. What type of substitution mechanism will be favored: SN1 or SN2? Draw the product that will result.arrow_forwardDraw the major product and mechanism for the following reactionsarrow_forwardFor each set of reactions, circle the mechanism (SN2 vs SN1), draw the main organic substitution/elimination product (for each reaction draw the product, though in some cases it may be equivalent) and indicate which reaction occurs at the faster rate.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
