
Concept explainers
(a)
Interpretation:
Complete
Concept introduction:
A bimolecular nucleophilic substitution (
Answer to Problem 8.43P
Complete
(i)
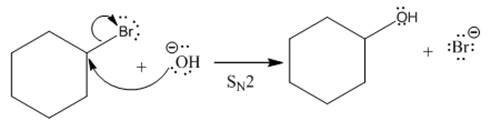
(ii)
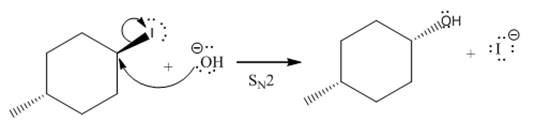
(iii)
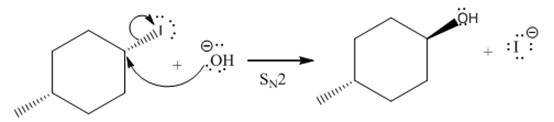
(iv)
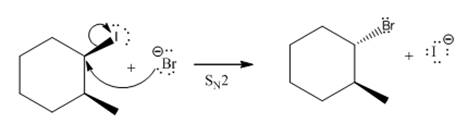
(v)
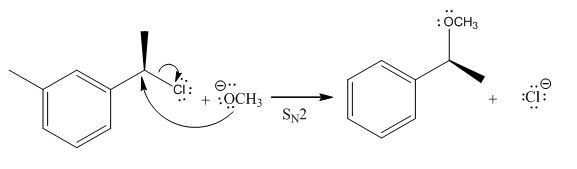
Explanation of Solution
(i)
The given reaction is
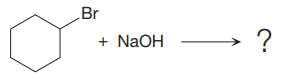
In the above reaction,
(ii)
The given reaction is
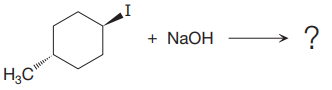
In the above reaction,
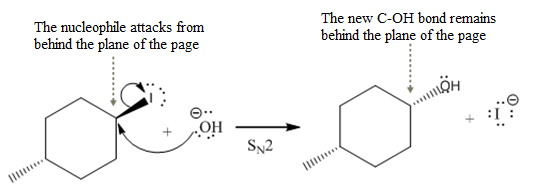
The stereochemistry of another group
(iii)
The given reaction
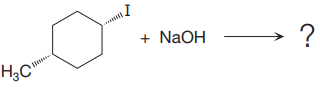
In the above reaction,
The stereochemistry of another group
(iv)
The given reaction
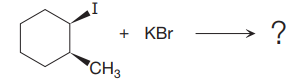
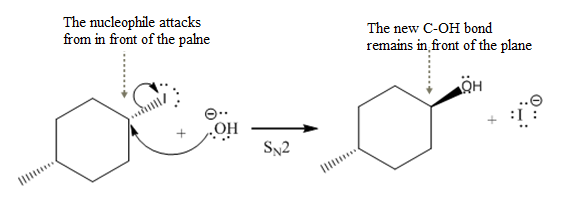
In the above reaction,
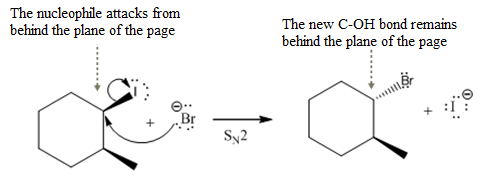
The stereochemistry of another group
(v)
The given reaction
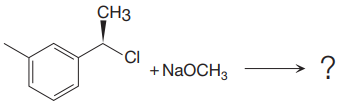
In the above reaction,
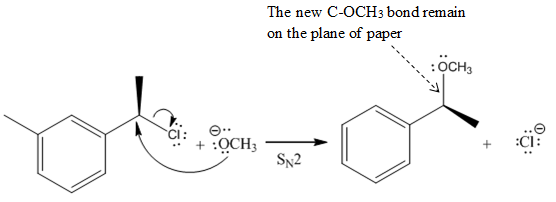
Another group
The Product of
(b)
Interpretation:
Complete
Concept introduction:
A unimolecular nucleophilic substitution (
If
Answer to Problem 8.43P
Complete
(i)
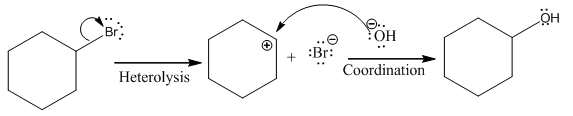
(ii)
(iii)
(iv)
(v)
Explanation of Solution
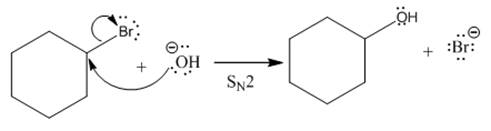
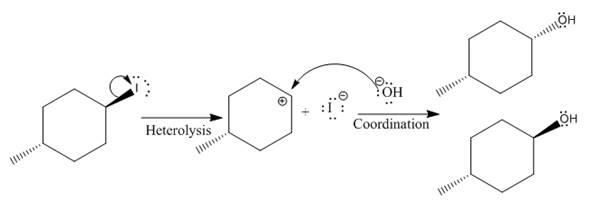
(i)
The given reaction is
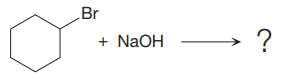

The leaving group in the above reaction is present on the plane of the planar, no stereochemistry is mentioned. Therefore, stereochemistry is not concerned at C, and the only isomer formed is the one shown in the above diagram.
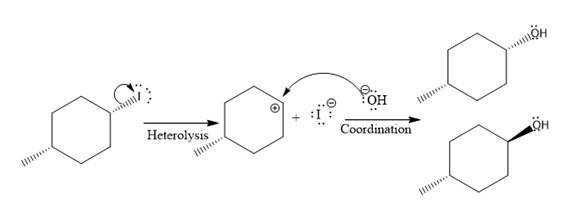
(ii)
The given reaction is
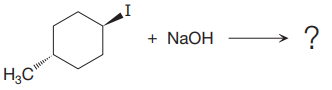
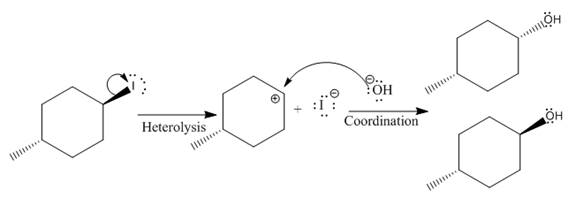
In the above reaction, leaving group (I) is present above the plane of the paper, thus the nucleophile attacks from both sides of the paper- behind the paper and in front of the paper resulting in the formation of two stereoisomers as shown above.
(iii)
The given reaction
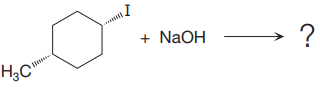

In the above reaction, leaving group (I) is present behind the plane of the paper, thus the nucleophile attacks from both sides of the paper- behind the paper and in front of the paper resulting in the formation of two stereoisomers as shown above.
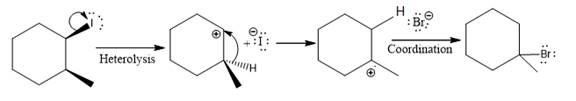
(iv)
The given reaction
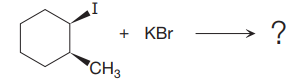
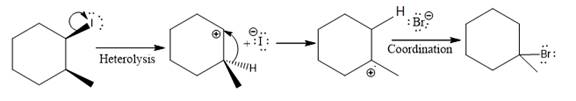
In the above reaction, the carbocation is stabilized by proton transfer. The new carbocation formed at the adjacent carbon from the leaving group is a more stable carbocation. The stereochemistry at the carbon (more stable carbocation) is of no concern. Therefore, the only isomer formed is as shown above.
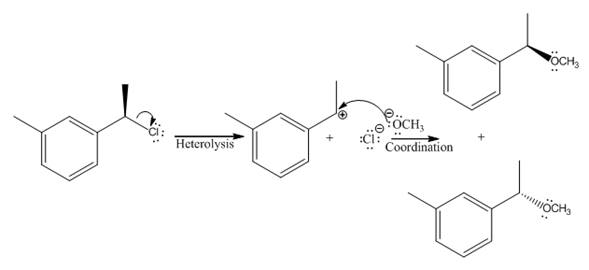
(v)
The given reaction
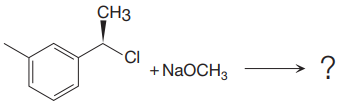
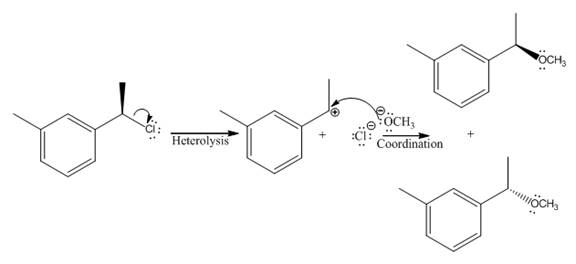
In the above reaction, chiral carbocation is formed once the leaving group leaves. Therefore, the nucleophile can attack from either side- from behind the paper and in front of the paper resulting in the formation of two stereoisomers as shown above.
Product of
Want to see more full solutions like this?
Chapter 8 Solutions
ORGANIC CHEMISTRY E-BOOK W/SMARTWORK5
- 2 a) Draw the complete, detailed mechanism for the following reaction, assuming that it takes place by an SN2 mechanism. b) What is the absolute configuration for each molecule shown in a)?arrow_forwardCan you please show me full detailed mechanism of this reaction? Please include formal charges, intermediates, and electron arrows.arrow_forwardCan you provide a mechanism for this reaction to show the major diastereoisomer that would be formed?arrow_forward
- Draw the complete, detailed E1 mechanism for each of the following reactions, and show all resonance structures, where applicable.arrow_forwardDraw all of the products and the mechanism for the reactions below.arrow_forwardSelect one deactivator & meta director to be on a benzene ring to start. You will then add a bromine to this group showing the COMPLETE mechanism, including all resonance structures. Put a box around the stabilizing resonance structure and explain why it is stabilizingarrow_forward
- Please draw the complete mechanism for the reaction belowarrow_forwardPlease show the mechanism and product for this reactionarrow_forwardComplete all the reactions/show the products, and for each reaction clearly and thoroughly explain which mechanism (E1, E2, SN1, SN2) is predominant and how it effects the product formation.arrow_forward
- Draw this compound in a chair form so that an E2 reaction is possible. Then, draw the mechanism and the major product. Your solution and structures must clearly show the 3D nature of the E2 mechanism.arrow_forwardComplete the following E2 mechanism. You only need to draw the mechanism for oneof the products, but please draw both products that would be formed.arrow_forwardA student proposes the following reaction mechanism for the reaction in Model 6. Which step inthis mechanism is least favorable? Explain your reasoning.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
