
Concept explainers
(a)
Interpretation:
Using curved arrow notation, an
Concept introduction:
The
Answer to Problem 8.1P
Mechanism for the given
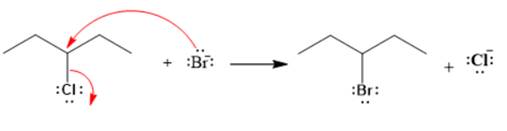
Explanation of Solution
The given reaction is
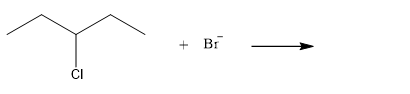
The given substrate is a secondary alkyl halide. Chlorine is a moderately good leaving group. Bromide ion is a strong nucleophile. An 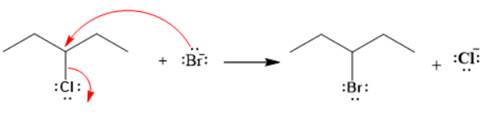
In the above reaction, the bromide ion attacks the carbon, to which the leaving group, chlorine, is attached from the side opposite the leaving group. In the product, the chlorine atom is replaced by a bromine atom.
The
(b)
Interpretation:
Using curved arrow notation, an
Concept introduction:
The
Answer to Problem 8.1P
Mechanism for the given
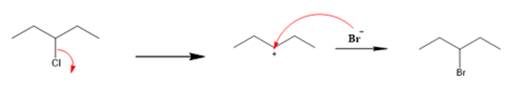
Explanation of Solution
The given reaction is
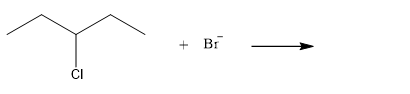
The first step is the breaking of carbon-chlorine bond. This will generate a secondary carbocation intermediate. In the second step, the electron rich
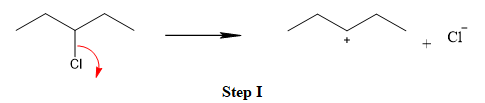
The leaving group is lost and forms a carbocation as follows:
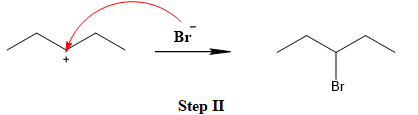
The
Want to see more full solutions like this?
Chapter 8 Solutions
ORG.CHEM W/TEXT+SOLU.MANUAL
- Submit the mechanism for the following reaction. Use the mechanism you draw to match this reaction with the correct energy diagram.arrow_forwardDraw a mechanism for the reaction of methylamine with formic acid. In the box to the left, draw any necessary curved arrows. Show the products of the reaction in the box to the right. Include any nonzero formal charges and all lone pairs of electrons. Finally, check the box to indicate which side of the reaction is favored at equilibrium.arrow_forwardDraw a curved arrow mechanism for the reaction. You can assume that all reactants and products are shown.arrow_forward
- Hello, I do not understand these questions and I am stuck. May I get help please?? Question: Draw the curved arrow mechanism for the following reactionarrow_forwardDraw the curved mechanism arrow* that shows the deprotonation of cyclohexanol by Na and draw the major organic product.*Only one arrow is needed, the arrow showing the electrons in the O-H bond going onto the oxygenarrow_forward. Use to curved arrow notation, propose a mechanism for the following reaction and state whether it is either SN1, E1, SN2, or E2. Give the IUPAC names of all organic reactants and products.arrow_forward
- Hello, I do not understand these questions for chemistry and I am stuck. May I get help please please?? An explanation leading to steps would be helpful. Thank you. Question: Draw the curved arrow mechanism for the following reaction.arrow_forwardDraw a mechanism for the following reaction: Draw all missing reactants and/or products in appropriate boxes by placing atoms on a box and connecting them with bonds. Add charges where needed. Electron-flow arrows should start on the electron(s) of an atom or a bond and should end on an atom, bond, or location where a new bond should be created.arrow_forwardDraw the mechanism and the major organic product for each of the following reactions. Please show all work with arrowsarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





