
Concept explainers
(a)
Interpretation:
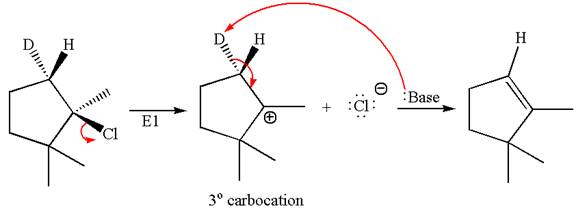
The detailed mechanism for the given reaction occurring via
Concept introduction:
The deuterium
Answer to Problem 8.64P
The
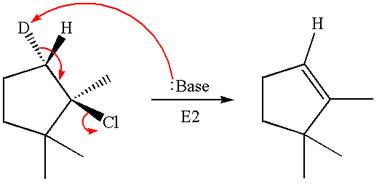
Explanation of Solution
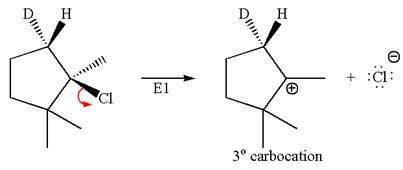
The given reaction equation is
Here, the chlorine atom is the leaving group, which can be eliminated along with either
In the given substrate, the leaving group
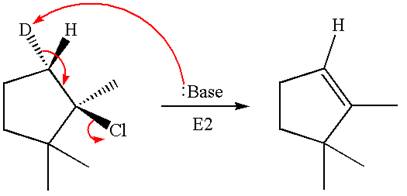
The product formed for the given reaction from
(b)
Interpretation:
The detailed mechanism for the given reaction occurring via
Concept introduction:
The deuterium
Answer to Problem 8.64P
The
Elimination of
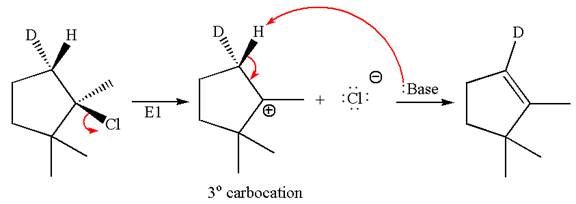
Elimination of
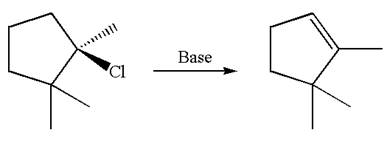
Explanation of Solution
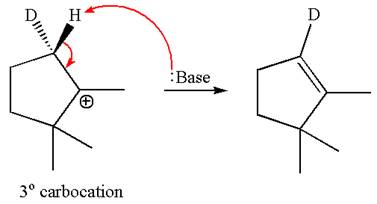
The given reaction equation is
Here, the chlorine atom is the leaving group, which can be eliminated along with either
According to
In the second step, both
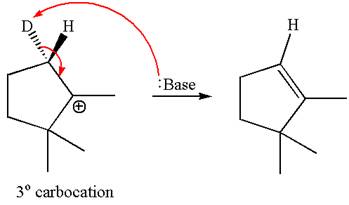
The product formed for the given reaction from
(c)
Interpretation:
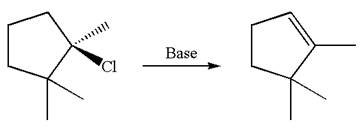
The molar mass of each product from
Concept introduction:
The molar mass is the sum of the
The
Answer to Problem 8.64P
The molar masses of the products formed by
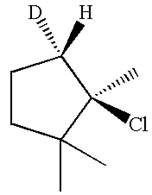
Explanation of Solution
The given reaction equation is
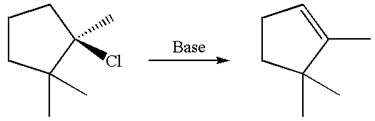
Here, the chlorine atom is the leaving group, which can be eliminated along with either
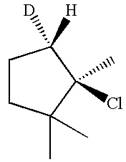
In the given substrate, the leaving group
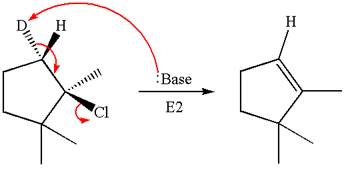
The molecular formula for this product is
According to
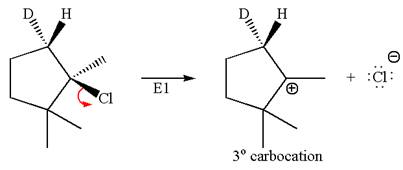
In the second step, both
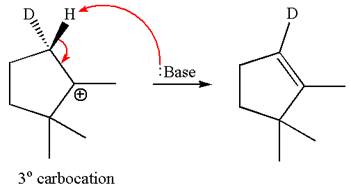
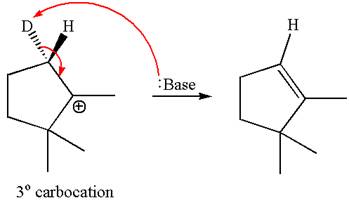
The molecular formulae for both the products formed by
Hence the molar mass of the product
The molar masses of each product formed by
Want to see more full solutions like this?
Chapter 8 Solutions
ORG.CHEM W/TEXT+SOLU.MANUAL
- A student proposes the following reaction mechanism for the reaction in Model 6. Which step inthis mechanism is least favorable? Explain your reasoning.arrow_forwardBetween E1 and E2, which reaction mechanism is more efficient to synthesize the (E) stereoisomer of this product on a large scale?arrow_forwardCould someone help me rank these in order of increasing reaction in an SN1 reaction? Thank you!arrow_forward
- Does the reaction likely proceed by the SN1, SN2, E1, or E2 mechanism?arrow_forwardConsider the following reaction being performed with a low concentration. Think about what type of substitution mechanism will be favored, SN2 or SN1, and what product will result.arrow_forwardDraw the major organic SN1 product for the reaction shown.arrow_forward
- please get the e1 and e2 product of this reactionarrow_forwardDraw the major organic product of the following reaction (see image), and select the mechanism which would dominate (SN1, SN2, E1, or E2).arrow_forwardis this an E1 or E2 mechanism for this reaction? What is the major product and mechanism for it?arrow_forward
- Draw arrows to show a typical SN1, SN2, E1 and E2 reactionarrow_forwardDraw the product of the following SN1 reactions, note the relationship between products if more than one forms:arrow_forwardFor each of the following reactions diagram the steps from the substrate to the product. For each step, a) over the reaction arrow supply the needed reagents b) name the type of reaction/mechanism {ROS (SN1, SN2, radical) E(E1, E2) A [acid-base, addition (carbocation, onium bridge, radical)]}arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
