
(a)
Interpretation:
The mechanism for the given elimination reaction including carbocation rearrangement is to be drawn.
Concept introduction:
The
Answer to Problem 8.65P
The
Explanation of Solution
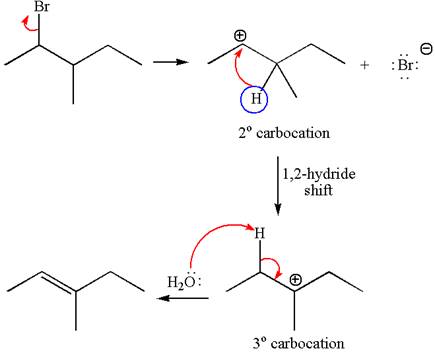
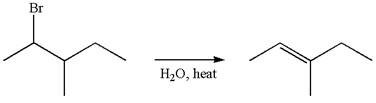
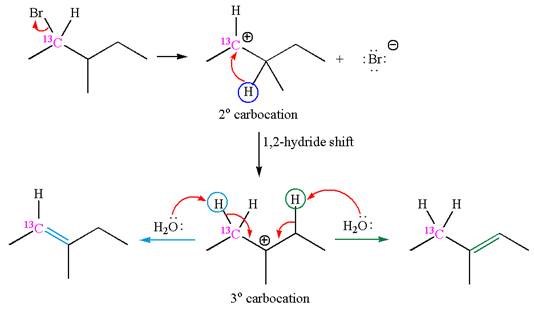
The given reaction equation is
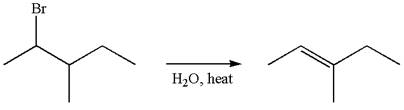
In first step, the leaving group
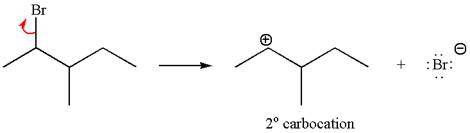
The carbocation formed is rearranged by
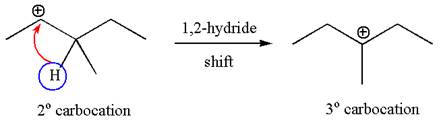
The water molecule acts as a base and abstracts a proton from the carbon adjacent to the carbocation, forming
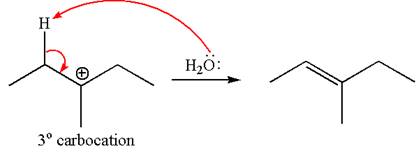
The mechanism for the given elimination reaction is drawn to show the carbocation rearrangement by
(b)
Interpretation:
The mechanism for the given elimination reaction without carbocation rearrangement is to be drawn.
Concept introduction:
The
Answer to Problem 8.65P
The
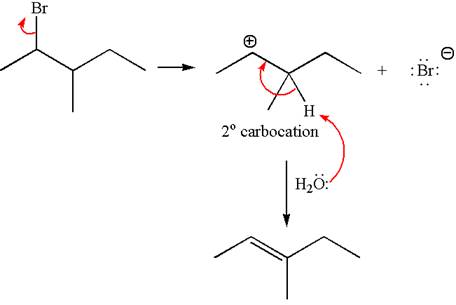
Explanation of Solution
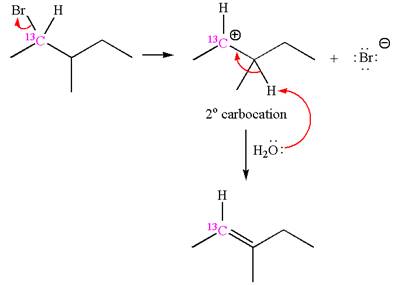
The given reaction equation is
In the first step, the leaving group
In the second step, without rearrangement, the proton is eliminated by the base
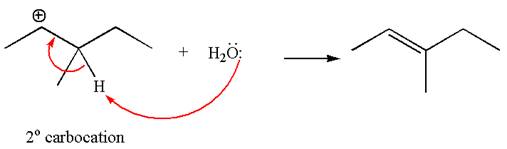
The mechanism for the given elimination reaction is drawn to without rearrangement step, indicating that the same product is formed with or without rearrangement.
(c)
Interpretation:
It is to be explained how the
Concept introduction:
The
Answer to Problem 8.65P
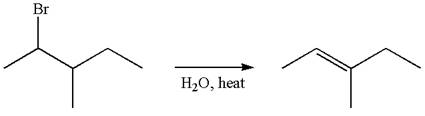
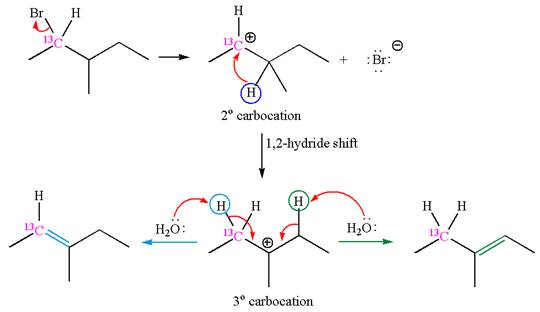
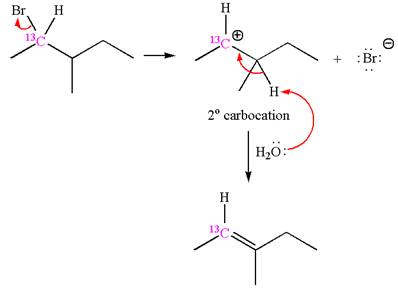
The reaction with carbocation rearrangement gave two products, and the reaction without carbocation rearrangement gave only one product, as shown below, indicating that the
Reaction with rearrangement:
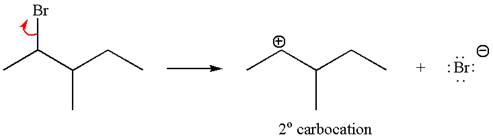
Reaction without rearrangement:
Explanation of Solution
The given reaction equation is
If the carbon bonded to the leaving group in the given substrate is labelled as
In one product, one of the double bonded carbon is
If the reaction proceeds without rearrangement, then only one product is formed where one of the double bonded carbon is
As the reaction with rearrangement of carbocation formed two products, and reaction without rearrangement formed only one product, it indicates that the E1 products depend on whether the rearrangement occurred.
It is explained that the E1 products depend on whether the reaction includes carbocation rearrangement occurring with
(d)
Interpretation:
How the deuterium isotope labeling is useful to determine whether the rearrangement is occurred in given
Concept introduction:
The
Answer to Problem 8.65P
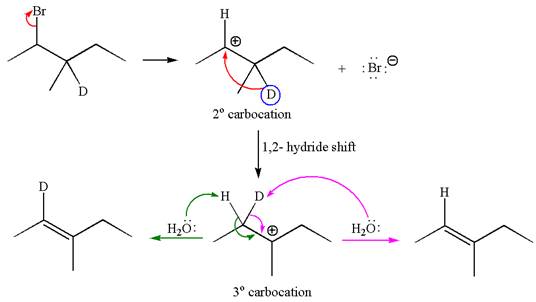
The reaction with carbocation rearrangement by migration of deuterium gave two products, and the reaction without carbocation rearrangement gave only one product as shown below, indicating that the deuterium isotope labeling is useful to determine whether the rearrangement occurred in the given
Reaction with rearrangement:
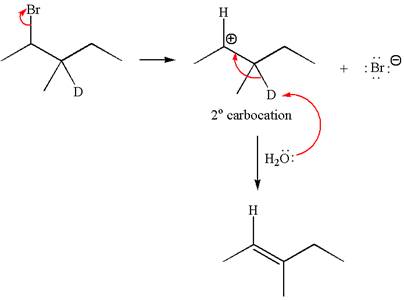
Reaction without rearrangement:
Explanation of Solution
The given reaction equation is
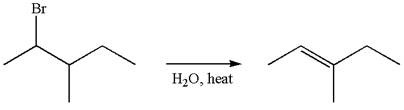
If the migrating hydrogen in the given substrate is replaced with deuterium, then two products are formed when the reaction occurred through carbocation rearrangement. The detailed mechanism is as follows:
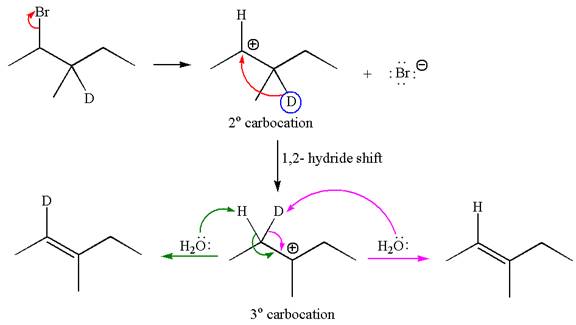
One product is formed by elimination of hydrogen atom and another by elimination of deuterium atom.
If the reaction proceeds without rearrangement, only one product is formed by elimination of deuterium atom. The detailed mechanism is as follows:
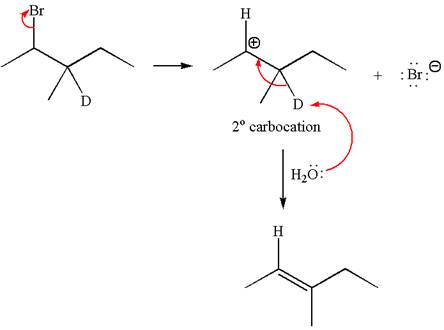
As the reaction with rearrangement of carbocation formed two products and reaction without rearrangement formed only one product, it indicates the E1 products depend on whether the rearrangement occurs.
It is explained on the basis of formation of different products that deuterium isotope labeling is useful to determine whether the rearrangement occurred in the given
Want to see more full solutions like this?
Chapter 8 Solutions
Organic Chemistry: Principles and Mechanisms (Second Edition)
- Identify the carbocations that are likely to rearrange, and state whether rearrangement will proceed via 1,2-methyl shift, or a 1,2-hydride shiftarrow_forwardBetween E1 and E2, which reaction mechanism is most efficient to synthesize the (E) stereoisomer of this product on an industrial scale?arrow_forwardGiven that an E2 reaction proceeds with anti periplanar stereochemistry, draw the products of each elimination. The alkyl halides in (a) and (b) are diastereomers of each other. How are the products of these two reactions related? Recall from Section 3.2A that C6H5 −is a phenyl group, a benzene ring bonded to another group.arrow_forward
- Given that an E2 reaction proceeds with anti periplanar stereochemistry, draw the products of each elimination. The alkyl halides in (a) and (b) are diastereomers of each other. How are the products of these two reactions related? Recall from Section 3.2A that C6H5– is a phenyl group, a benzene ring bonded to another group.arrow_forwardUse curved arrows to show how E is converted to F by a two-stepreaction sequence consisting of a [1,5] sigmatropic rearrangementfollowed by a [4 + 2] cycloaddition.arrow_forwardHow do you properly write an SN1 reaction, with transition states/intermediate steps included?arrow_forward
- Choose the compound below that gives a carbocation intermediate that undergoes rearrangement?arrow_forwarda. What are the products of following reaction?b. Write the reaction mechanism for each using the right arrows and define it as Sn2, Sn1, E2 or E1.c. Explain why do you choose that product and mechanism or in case something else happens explainwhy.arrow_forward(a) Give a mechanism for this reaction, showing how the two products arise as aconsequence of the resonance-stabilized intermediate.(b) The bromination of cyclohexene using NBS gives only one major product, as shown onthe previous page. Explain why there is no second product from an allylic shift.arrow_forward
- Illustrate the carbocation pathway for the following reaction below.arrow_forwardWhich rearrangement product will form preferentially during the [1,2]-rearrangementof an R-group? Motivate.arrow_forwardWrite the mechanism of the following bromination reaction and explain the regioselectivity of the bromination reaction. Draw the mechanism, transition state, energy diagram, etc. The same reaction with chlorine is not much regioselective in Q1. Explain the decreased regioselectivity of the following chlorination reaction by comparing the bromination reaction in Q1. Draw the mechanism, transition state, energy diagram, etc.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

