
Concept explainers
Predict the parent structures of the following molecules.
(a)
Interpretation:
The parent structure of
Explanation of Solution
The sum of atoms attached to the central metal atom and the unshared electron pairs is termed as electron domains. The electron domains are calculated by drawing the Lewis structure which results in the parent structure or geometry of the molecule.
There are two electron domains present in
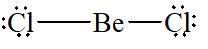
Thus, the parent structure of
(b)
Interpretation:
The parent structure of
Explanation of Solution
The sum of atoms attached to the central metal atom and the unshared electron pairs is termed as electron domains. The electron domains are calculated by drawing the Lewis structure which results to the parent structure or geometry of the molecule.
There are four electron domains present in the
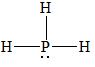
Thus, the parent structure of
(c)
Interpretation:
The parent structure of
Explanation of Solution
The sum of atoms attached to the central metal atom and the unshared electron pairs is termed as electron domains. The electron domains are calculated by drawing the Lewis structure which results in the parent structure or geometry of the molecule.
There are four electron domains present in
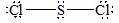
Thus, the parent structure of
(d)
Interpretation:
The parent structure of
Explanation of Solution
The sum of atoms attached to the central metal atom and the unshared electron pairs is termed as electron domains. The electron domains are calculated by drawing the Lewis structure which results in the parent structure or geometry of the molecule.
There are three electron domains present in the
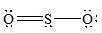
Thus, the parent structure of
(e)
Interpretation:
The parent structure of
Explanation of Solution
The sum of atoms attached to the central metal atom and the unshared electron pairs is termed as electron domains. The electron domains are calculated by drawing the Lewis structure which results in the parent structure or geometry of the molecule.
There are four electron domains present in
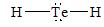
Thus, the parent structure of
(f)
Interpretation:
The parent structure of
Explanation of Solution
The sum of atoms attached to the central metal atom and the unshared electron pairs is termed as electron domains. The electron domains are calculated by drawing the Lewis structure which results in the parent structure or geometry of the molecule.
There are four electron domains present in
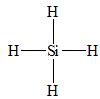
Thus, the parent structure of
(g)
Interpretation:
The parent structure of
Explanation of Solution
The sum of atoms attached to the central metal atom and the unshared electron pairs is termed as electron domains. The electron domains are calculated by drawing the Lewis structure which results in the parent structure or geometry of the molecule.
There are three electron domains present in the
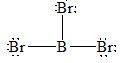
Thus, the parent structure of
(h)
Interpretation:
The parent structure of
Explanation of Solution
The sum of atoms attached to the central metal atom and the unshared electron pairs is termed as electron domains. The electron domains are calculated by drawing the Lewis structure which results in the parent structure or geometry of the molecule.
There are four electron domains present in the
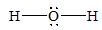
Thus, the parent structure of
Want to see more full solutions like this?
Chapter 8 Solutions
INTRODUCTION TO CHEMISTRY-ACCESS
- how would I identify which of these Lewis structures (A, B, C or D) is correct for PO33- and what is the VSEPR shape of the compound?arrow_forwardCompounds such as NaBH4, Al(BH4)3, and LiAlH4 are complexhydrides used as reducing agents in many syntheses.(a) Give the oxidation state of each element in these compounds.(b) Write a Lewis structure for the polyatomic anion in NaBH4, and predict its shape.arrow_forwardWhy are there 2 NH3+ in the structure?arrow_forward
- 18 For the compound V(ClO)5 what is the charge on vanadium?arrow_forwardwhat is the shape of [Co(NH3)6]^3+ ?arrow_forwardIn many ways, arsenate (AsO4 3−) is very similar to phosphate (PO4 3-), yet it does not substitute for phosphate in biomolecules. After reviewing the essential atomic characteristics of the element arsenic, explain this phenomenon.arrow_forward
- 3L5.2arrow_forwardStarting with CnH2n+2, derive the general formula for the compounds shown:arrow_forwardTrue or false: (a) The C¬C bonds in benzene are all the samelength and correspond to typical single C¬C bond lengths.(b) The C¬C bond in acetylene, HCCH, is longer than theaverage C¬C bond length in benzene.arrow_forward
- What is the IHD of a chemical compound with a formula of C6H5NO2? a 4 b 5 c 6 d 7arrow_forwardWhich property of the third-row nonmetallic elementsmight be the one depicted below: (a) first ionizationenergy, (b) atomic radius, (c) electronegativity, (d) meltingpoint, (e) X¬X single-bond enthalpy?arrow_forwardCompounds such as NaBH4, Al(BH4)3, and LiAlH4 are complex hydrides used as reducing agents in many syntheses. (a) Give the oxidation state of each element in these compounds. (b) Write a Lewis structure for the polyatomic anion in NaBH4, and predict its shape.arrow_forward
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning





