
Concept explainers
a.
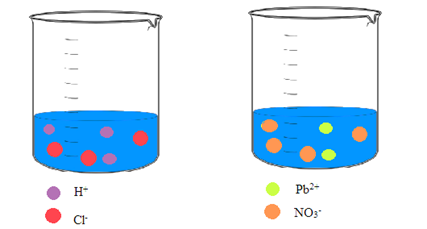
Interpretation: A picture of each solution needs to be drawn showing the ions in an aqueous solution of hydrochloric acid and an aqueous solution of lead(II) nitrate.
Concept Introduction: Ionic compounds on dissolving in water result in a solution that contains the separated ions. Due to the polar nature of water, the ions are attracted to the molecules of water.
a.
Answer to Problem 3RQ
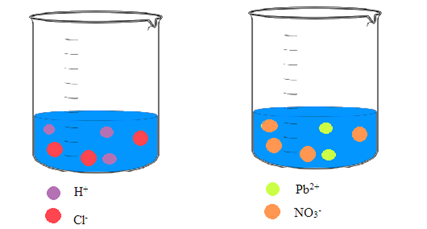
Explanation of Solution
Hydrochloric acid and lead(II) nitrate are ionic compounds that means these compounds are formed by complete transfer of electron(s). The chemical formula of hydrochloric acid and lead(II) nitrate is
From the dissociation reaction it is clear that 1 mole of
b.
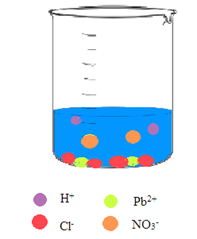
Interpretation: A picture needs to be drawn when the aqueous solution of hydrochloric acid and aqueous solution of lead(II) nitrate are mixed.
Concept Introduction: A reaction in which there is a formation of an insoluble salt due to the mixing of solutions containing soluble salts is said to be precipitation reactions.
b.
Answer to Problem 3RQ
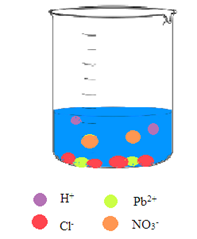
Explanation of Solution
The
So, the pictorial representation after mixing the two aqueous solutions of hydrochloric acid and lead(II) nitrate is:
c.
Interpretation: The products formed on mixing aqueous solution of hydrochloric acid and aqueous solution of lead(II) nitrate should be determined.
Concept Introduction: A reaction in which there is a formation of an insoluble salt due to the mixing of solutions containing soluble salts is said to be precipitation reactions.
c.
Answer to Problem 3RQ
The products forme are:
Solid lead chloride,
Explanation of Solution
The chemical reaction that will take place on mixing aqueous solution of hydrochloric acid and aqueous solution of lead(II) nitrate will be precipitation reaction as this reaction will lead to the formation of insoluble salt, lead chloride and aqueous nitric acid. The complete balanced reaction for the process is:
Hence, the products forme are:
Solid lead chloride,
d.
Interpretation: The net ionic equation for the reaction taken place on mixing aqueous solution of hydrochloric acid and aqueous solution of lead(II) nitrate should be determined.
Concept Introduction: The reaction that involves only the participating ions in the reaction is said to be the net ionic equation.
d.
Answer to Problem 3RQ
Explanation of Solution
The chemical reaction that will take place on mixing aqueous solution of hydrochloric acid and aqueous solution of lead(II) nitrate will be precipitation reaction as this reaction will lead to the formation of insoluble salt, lead chloride and aqueous nitric acid. The complete balanced reaction for the process is:
The total ionic equation for the above reaction is:
In the total ionic equation
Chapter 8 Solutions
World of Chemistry
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





