
ORGANIC CHEMISTRY-WILEYPLUS NEXTGEN
4th Edition
ISBN: 9781119760924
Author: Klein
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Question
Chapter 8.8, Problem 21ATS
Interpretation Introduction
Interpretation: The structure of both the product and major diastereomer should be determined based on the below reaction.
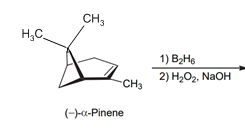
Concept introduction:
The acid-catalyzed hydration, hydroboration-oxidation, oxymercuration-demercuration, etc. are some examples of addition reactions of
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Sharpless epoxidation can be used to stereoselectively convert an allylic alcohol to an
epoxy alcohol using Ti(O/Pr)4, t-butyl hydroperoxide (TBHP) and a chiral diethyl tartrate
(DET), as exemplified in the formation of compound E below.
HO
OBn
Ti(O/Pr)4
TBHP
L-(+)-DET
CH₂Cl₂
HO
E
OBn
(i) Assign the configuration of the two chiral centres in compound E using CIP rules.
(ii)
E is produced in 94% ee under the conditions. What is the percentage of each
enantiomer?
(iii)
E can be readily debenzylated to give a diol structure, is the diol chiral? Explain.
Several diamines are building blocks for the synthesis of pharmaceuticals and agro-
chemicals. Show how both 1,3-propanediamine and 1,4-butanediamine can be pre-
pared from acrylonitrile.
NH2
H,N
`NH2
H,N
CH,=CH-C=N
1,3-Propanediamine
1,4-Butanediamine
Acrylonitrile
A chemist allows some pure (2S,3R)-3-bromo-2,3-diphenylpentane to react with a solution of sodium ethoxide(NaOCH2 CH3) in ethanol. The products are two alkenes: A (cis-trans mixture) and B, a single pure isomer. Under the same conditions, the reaction of (2S,3S)-3-bromo-2,3-diphenylpentane gives two alkenes, A (cis-trans mixture) and C. Upon catalytic hydrogenation, all three of these alkenes (A, B, and C) give 2,3-diphenylpentane. Determine the structures of A, B, and C; give equations for their formation; and explain the stereospecificity of these reactions.
Chapter 8 Solutions
ORGANIC CHEMISTRY-WILEYPLUS NEXTGEN
Ch. 8.3 - Provide a systematic name for each of the...Ch. 8.3 - Prob. 2CCCh. 8.3 - Prob. 3CCCh. 8.3 - Prob. 4CCCh. 8.5 - Prob. 5CCCh. 8.5 - Prob. 6CCCh. 8.5 - Prob. 1LTSCh. 8.5 - Prob. 7PTSCh. 8.5 - Prob. 8ATSCh. 8.5 - Prob. 9CC
Ch. 8.5 - Prob. 2LTSCh. 8.5 - Prob. 10PTSCh. 8.5 - Prob. 11ATSCh. 8.6 - Prob. 12CCCh. 8.6 - Prob. 13CCCh. 8.6 - Prob. 3LTSCh. 8.6 - Prob. 14PTSCh. 8.6 - Prob. 15ATSCh. 8.7 - Predict the product for each reaction, and predict...Ch. 8.7 - Prob. 17CCCh. 8.8 - Prob. 18CCCh. 8.8 - Prob. 19CCCh. 8.8 - Prob. 4LTSCh. 8.8 - Prob. 20PTSCh. 8.8 - Prob. 21ATSCh. 8.9 - Prob. 5LTSCh. 8.9 - Prob. 22PTSCh. 8.9 - Prob. 23ATSCh. 8.10 - Prob. 24CCCh. 8.10 - Prob. 6LTSCh. 8.10 - Prob. 25PTSCh. 8.10 - Prob. 26ATSCh. 8.10 - Prob. 27ATSCh. 8.11 - Prob. 7LTSCh. 8 - Prob. 47PP
Knowledge Booster
Similar questions
- Cembrene, C20H32, is a diterpenoid hydrocarbon isolated from pine resin. Cembrene has a UV absorption at 245 nm, but dihydrocembrene (C20H34), the product of hydrogenation with 1 equivalent of H2, has no UV absorption. On exhaustive hydrogenation, 4 equivalents of H2 react, and octahydrocembrene, C20H40, is produced. On ozonolysis of cembrene, followed by treatment of the ozonide with zinc, four carbonylcontaining products are obtained: Propose a structure for cembrene that is consistent with its formation from geranylgeranyl diphosphate.arrow_forwardQ. Identify the products (from 1 to 10) in the following scheme: CI HNO3 1 2 AICI3 H,SO4 Zn(Hg) HCI KMNO4 (hot, concd.) 4 3 Br2 |hv (CH3),CO¯ K* Mg, ether Br2 5 C„H14B12 CH;COCH3 NaOCH3 PHCO3H Fused KOH, 200°C 10 8 Alcohol (3º) An Ether Epoxide derivative 2 5 6 8 9 10arrow_forwardArrange the following groups in increasing CIP priority order: * -C-OCH3 С-ОН C–CH3 -C-NH2 I II III IV III, IV, II, I O II, I, IV, II O II, III, I, IV O I, I, IV, Iarrow_forward
- A 1.62 M solution of (R)-2-butanol is mixed with an equal volume of a 0.810 M solution of racemic 2-butanol, and the resulting solution is analyzed in a sample container that is 1 dm long. What observed rotation is expected? The specific rotation of (R)-2-butanol is –13.9 degrees mL g- dm-'. a = degarrow_forwardpoints A chemist allows some pure (2S, 3R)-3-bromo-2,3-diphenylpentane to react with a solution of sodium ethoxide (NaOCH2CH3) in ethanol. The products are two alkenes: A (Cis-trans mixture) and B, a single pure isomer. Under the same conditions, the reaction of (2S, 35)-3-bromo-2,3- diphenylpentane gives two alkenes. A (Cis-trans mixture) and C. Upon catalytic hydrogenation, all three of these alkenes (A, B and C) give 2,3- diphenylpentane. Determine the structures of A, B and C, give equations for their formation, and explain the stereospecificity of these reactions. Click here to have access to www.autodraw.com/ For the toolbar, press ALT+F10 (PC) or ALT+FN+F10 (Mac).arrow_forward+ H3C- CH₂ - CH2 - COR and have undergone McLafferty rearrangement and Ortho effect respectively. What are the possible fragments obtained as a result of these processes ? COOCH3 OH + (A) [R-CH₂-C (A) R-CH₂-CH₂ - CH₂ - COH @E OH]: [c C6H4 O and CO (B) H₂C=CROH and H₂C = CH₂ [2] : [ C₂H4O3 and CH4 (C) (D) H₂CCROH and C₂H4; [(C6H4O), CO and CH₂OH] RH, C₂H4 and CH₂CO; [(C₂H4O), CO and CH₂OH]arrow_forward
- Give a rough estimate for delta H of the Pt-catalyzed hydrogenation of 1,2 dimethylcyclohexene. And draw the HOMO orbital of H2 and overlay it with the LUMO of the alkenearrow_forward1. Predict the major product(s) for the following reactions. Indicate stereochemistry and draw enantiomers when appropriate. Show stereochemistry with two lines in the plane and a wedge and/or dash at the chirality center. No reaction mechanisms are necessary. (a) :0: (b) :0: 1. H₂O €0. Na:QH 2. H+, H₂O 1. H₂O ee.. Na:OH 2. H+, H₂O 2. (a) Copy the reaction scheme for the ester hydrolysis of methyl salicylate (see Scheme 7), make a table showing the physical properties of methyl salicylate, 3.0 M NaOH and salicylic acid, and (b) outline the experimental procedure in your laboratory notebook. Reaction NaOH(aq) ONa HCl(aq) OH ONa Methyl salicylate OH OH Salicylic acid + NaCl + -OHarrow_forwardWhen the alkene A was treated first with Hg(OAc)2 in MeOH and second with NaBH4 the product was the ether B. Using curved arrows please give the mechanism for the first step of (Hg(OAc)2 in MeOH) this reaction, including any regioselectivity or stereoselectivity. H3C 1. Hg(OAc)2, MeOH H3C O-CH3 =CH2 ✓ -CH3 H3C 2. NaBH H3C A Barrow_forward
- Which of the following statements best describes the stereospecificity of the resultingproduct of oxidation of an alkene using KmNO4? *Hydroxyl groups are found on opposite side.Alcohol groups undergo bond rotation.Alcohol groups are transformed into acids.Hydroxyl groups are found on the same side.What is the expected product from the hydrohalogenation of hex-1-ene using HCl? *1-chlorohexane1-chlorohex-1-ene1,2-dichlorohexane2-chlorohexanearrow_forwardc. The biosynthesis of valine by bacteria involves the following sequence: (CH3)2C-CHCO2H (CH3)2CHCCO,H (CH3)2CHCHCO,H ОН ОН NH2 The stereochemistry of the reaction has been examined using the starting diol in which each methyl group was separately replaced by CD3. The diol-d, the 2R,3R configuration produces 2S,3S-valine-d, whereas the 2R,35 diol-d, produces 25,3R-valine-d3. From this information deduce whether the C(2) and C(3) hydroxy are replaced with inversion or retention of configuration. Show the basis for your conclusion. d. A synthesis of the important biosynthetic intermediate mevalonic acid starts with the enzymatic hydrolysis of the diester 14-C by pig liver esterase. The pro-R ester group is selectively hydrolyzed. Draw a three-dimensional structure of the product. of CH3 H3CO2CCH,CCH,CO2CH3 1. ОН 14-Carrow_forwardWhich stereoisomer of 3,4-dimethyl-3-hexene forms (3S,4S)-3,4-dimethylhexane and (3R,4R)-3,4-dimethylhexane when it reacts with H2, Pd/C?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning