
Concept explainers
(a)
Interpretation: The reaction of an alkene with dilute sulphuric acid in the presence of methanol as solvent occurs quite similar to acid-catalyzed hydration but in the second step alcohol acts as a nucleophile. The mechanism for the given chemical transformation is to be interpreted.
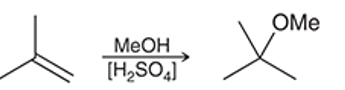
Concept introduction:
(b)
Interpretation: The mechanism for the given chemical transformation is to be interpreted.
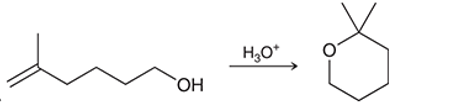
Concept introduction:
Alkenes are unsaturated hydrocarbons with at least one double bond between the carbon atoms. The acid-catalyzed hydration reactions of alkene involve the reaction of an alkene with
Want to see the full answer?
Check out a sample textbook solution
Chapter 8 Solutions
ORGANIC CHEMISTRY-WILEYPLUS NEXTGEN
- Compare the following reactions. Which is faster? Using a Free Energy Diagram, justify your choice. Br NaOH Br NaOHarrow_forwardDraw out the complete mechanism of the reaction and include all relevant electrons and arrows showing electron movement. NaOHarrow_forwardDraw a stepwise, detailed mechanism for the following reaction. CH3NH2 N-CH3 CH,NH, Cr (excess)arrow_forward
- Complete the following stepwise reaction mechanism problems based on the reaction conditions given: Draw the stepwise mechanism.arrow_forwardQ- mahesh Please draw out the mechanism and explain the reagents you use.arrow_forwardDescribe the following chemical reactions as SN1, SN2, E1 and W2. Draw a curved arrow mechanism for each reaction.arrow_forward
- Complete the reaction map by matching A-E with the given choices.arrow_forwardWhen a single compound contains both a nucleophile and a leaving group, an intramolecular reaction may occur. With this in mind, draw the product of the following reactionarrow_forwardLike other electrophiles, carbocations add to alkenes to form new carbocations, which can then undergo substitution or elimination reactions depending on the reaction conditions. With this in mind, draw a stepwise mechanism for the following reaction, which involves the addition of an electrophile—a carbocation—to a double bond.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
