
Concept explainers
Predict the products of the following reactions. Don’t worry about the size of the molecule; concentrate on the
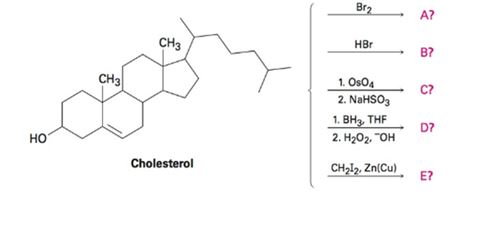
a)
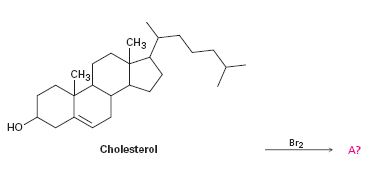
Interpretation:
To predict the product formed when cholesterol reacts with bromine.
Concept introduction:
The reaction of halogens to alkenes occurs with anti stereochemistry, that is, the two halogen atoms come from opposite faces of the double bond- one from top face and other from bottom face. In the first step the addition of halogen to the double bond in the alkenes results in the formation of a cyclic halonium ion with the simultaneous elimination of a halide ion. The large halonium ion shields one side of the molecule. Hence the attack of the halide ion occurs from the opposite, unshielded side to yield the trans product.
To predict:
The product formed when cholesterol reacts with bromine.
Answer to Problem 48AP
The product formed when cholesterol reacts with bromine.
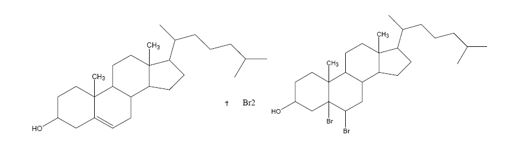
Explanation of Solution
In the first step, the addition of bromine to the double bond in cholesterol results in the formation of a cyclic bromonium ion with the elimination of a bromide ion. In the second step the bromide ion attacks the bromonium ion from the opposite, unshielded side to yield a dibromo derivative.
The product formed when cholesterol reacts with bromine.
b)
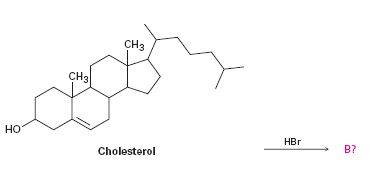
Interpretation:
To predict the product formed when cholesterol reacts with HBr.
Concept introduction:
The addition of hydrogen halides to alkenes takes place through the formation of a carbocation intermediate by the attack of the π electrons in the double bond on the positively polarized hydrogen of the hydrogen halide. The halogen is eliminated as a halide ion. In the second step, the halide ion reacts with the carbocation to yield the product.
To predict:
The product formed when cholesterol reacts with HBr.
Answer to Problem 48AP
The product formed when cholesterol reacts with HBr is
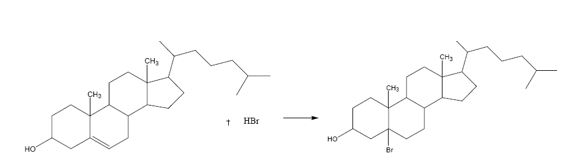
Explanation of Solution
The addition of hydrogen bromide to cholesterol takes place through the formation of a carbocation intermediate by the attack of the π electrons in the double bond on the positively polarized hydrogen of the hydrogen bromide. The bromine is eliminated as bromide ion which reacts in the second step with the carbocation to yield the product.
The product formed when cholesterol reacts with HBr is
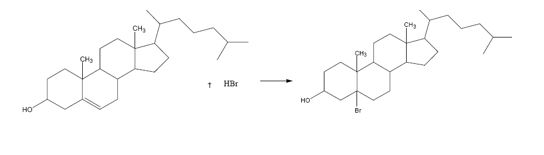
c)
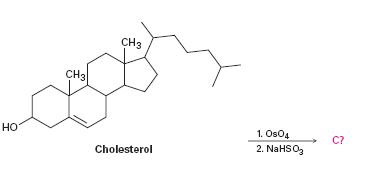
Interpretation:
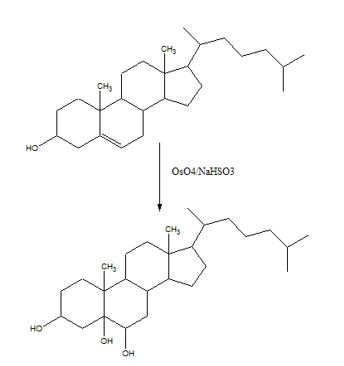
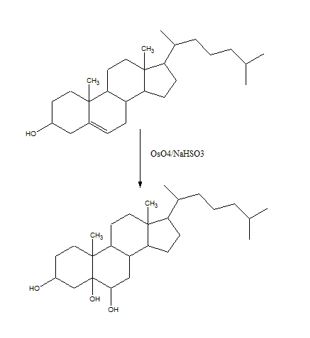
To predict the product formed when cholesterol reacts first with OsO4 and then with NaHSO3.
Concept introduction:
Hydroxylation of double bonds can be carried out directly by treating the alkene with osmium tetroxide, OsO4, in the presence of N-phenylmorpholine N-oxide. The reaction occurs with syn stereochemistry through the formation of a cyclic intermediate, called osmate, formed by the addition of OsO4 to the alkene in a single step. The cyclic osmate is then cleaved to give the cis-1, 2-diol by treatment with NaHSO3.
To predict:
The product formed when cholesterol reacts with first with OsO4 and then with NaHSO3.
Answer to Problem 48AP
The product formed when cholesterol reacts with first with OsO4 and then with NaHSO3 is
Explanation of Solution
The addition of OsO4 to the double bond in cholesterol takes place in a single step to give an osmate. The cyclic osmate when cleaved by treatment with NaHSO3 gives the cis-diol as the product.
The product formed when cholesterol reacts with first with OsO4 and then with NaHSO3 is
d)
Interpretation:
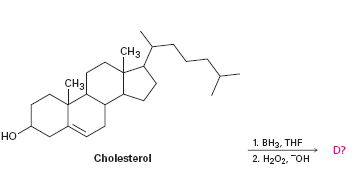
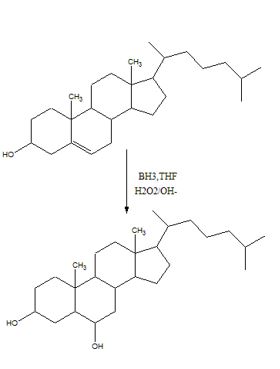
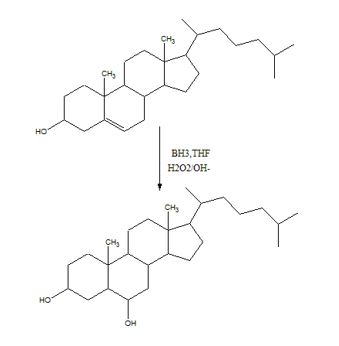
To predict the product formed when cholesterol reacts first with BH3, THF and then with H2O2, OH-.
Concept introduction:
The hydroboration-oxidation reaction takes place with syn stereochemistry and results in a non-Markovnikov addition of water to the double bond in the alkene. The resulting product has the hydroxyl group on the less highly substituted carbon.
To predict:
The product formed when cholesterol reacts with first with BH3, THF and then with H2O2, OH-.
Answer to Problem 48AP
The product formed when cholesterol reacts with first with BH3, THF and then with H2O2, OH- is
Explanation of Solution
During hydroboration, the addition of B-H to the double bond takes place with a syn stereochemistry. The boron atom being bigger than hydrogen gets attached to the less highly substituted carbon atom (less steric crowding). During the oxidation step, the boron is replaced by an –OH group with the same stereochemistry.
The product formed when cholesterol reacts with first with BH3, THF and then with H2O2, OH- is
e)
Interpretation:
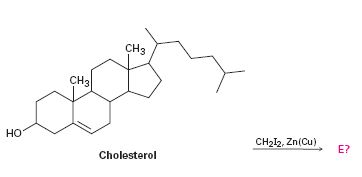
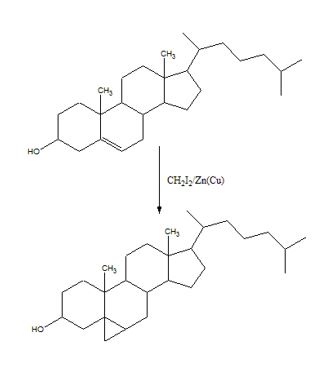
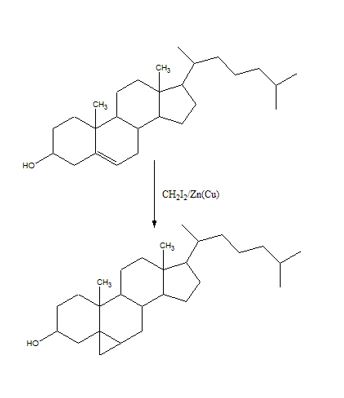
To predict the product formed when cholesterol reacts with CH2I2 in the presence of Zn (Cu).
Concept introduction:
The reaction given is an example of Simmons-Smith reaction. When CH2I2 is treated with Zn/Cu couple, iodomethylzinc iodide, ICH2ZnI, is formed. This ICH2ZnI transfers a CH2 group to the double bond in alkene to form a cyclopropane ring in the product.
To predict:
The product formed when cholesterol reacts with CH2I2 in the presence of Zn (Cu).
Answer to Problem 48AP
The product formed when cholesterol reacts with CH2I2 in the presence of Zn (Cu) is
Explanation of Solution
When CH2I2 is treated with Zn/Cu couple, iodomethylzinc iodide, ICH2ZnI, is formed. This ICH2ZnI transfers a CH2 group to the double bond in cholesterol to form a cyclopropane ring in the product.
The product formed when cholesterol reacts with CH2I2 in the presence of Zn (Cu) is
Want to see more full solutions like this?
Chapter 8 Solutions
ORGANIC CHEM.(LL)-W/OWL V2 >CUSTOM<
- Please provide the products produced from the following reaction.arrow_forwardPlease show arrows and step by step work on how you got the end product.arrow_forwardDetermine the type of reaction and the major product of the following reaction. Please explain the process leading up to your conclusion.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





