
Concept explainers
Interpretation:
A reactant that could be reacts with hexachlorocylopentadiene to gives chlordane through the Diels-Alder reaction has to be given.
Concept Introduction:
Cycloaddition reaction is a concerted addition reaction of two reactants to form a ring; in which two π-bonds are converted to two σ-bonds.
Diels-Alder reaction is a
The mechanism of the ring formation in the Diels-Alder reaction is drawn by using three arrows representing the shifting of π-bonds in the two π-systems in a clockwise or counter clockwise fashion, so that the ring formation will take place.
Cyclopentadiene can undergo Diels-Alder reaction itself to form a dimer,
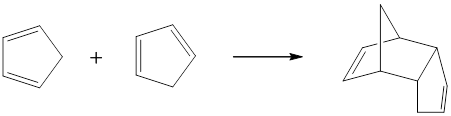
Endo-product is the major product in bicyclic-products of Diels-Alder reaction; because the electron-withdrawing substituents of dienophile and the newly forming π-bond of diene are interacted each other.
Want to see the full answer?
Check out a sample textbook solution
Chapter 9 Solutions
Organic Chemistry
- Bicyclo-2,5-heptadiene can be prepared in two steps from cyclopentadiene and vinyl chloride. Provide a mechanism for each step.arrow_forwardWhich of the following is most soluble in basic medium? a. cyclopropane b. 1,3-cyclobutadiene c. 1,3-cyclopentadiene d. benzenearrow_forward1,4-Pentadiene (CH2=CH-CH2-CH=CH2) is a liquid at room temperature and has a density of 0.66 g/mL and molar mass of 68.12 g/mol. In a laboratory experiment, 3.80 mL of this compound was treated with 4.80 mL of conc. H2SO4 (100% w/w; molar mass 98.08 g/mol). Note that the density of conc. H2SO4 is 1.84 g/mL. The resulting sulfate ester was then treated with 1.20 mL of water (molar mass 18.02 g/mol) affording, after work- up, 2,4-pentanediol (molar mass 104.15 g/mol) as the crude product. The crude product was then purified by simple distillation, which yielded 2.00 g of pure product. What is the theoretical yield of 2,4-pentanediol expressed in grams? Show calculations. What is the percentage yield of pure 2,4-pentanediol?arrow_forward
- 1,4-Pentadiene (CH2=CH-CH2-CH=CH2) is a liquid at room temperature and has a density of 0.66 g/mL and molar mass of 68.12 g/mol. In a laboratory experiment, 3.80 mL of this compound was treated with 4.80 mL of conc. H2SO4 (100% w/w; molar mass 98.08 g/mol). Note that the density of conc. H2SO4 is 1.84 g/mL. The resulting sulfate ester was then treated with 1.20 mL of water (molar mass 18.02 g/mol) affording, after work- up, 2,4-pentanediol (molar mass 104.15 g/mol) as the crude product. The crude product was then purified by simple distillation, which yielded 2.00 g of pure product. a. Provide a balanced chemical equation to show the reaction between 1,4-pentadiene and sulfuric acid. Do not use molecular formulas in the chemical equation except for sulfuric acid. b. What reactant is the limiting reagent in this chemical equation? Show calculations to support your answer.arrow_forwardα-Terpinene, C10H16, is a pleasant-smelling hydrocarbon that has been isolated from oil of marjoram. On hydrogenation over a palladium catalyst, α-terpinene reacts with 2 molar equivalents of H2 to yield a hydrocarbon, C10H20. On ozonolysis, followed by reduction with zinc and acetic acid, α-terpinene yields two products, glyoxal and 6-methyl-2,5- heptanedione. (a) How many degrees of unsaturation does a-terpinene have? (b) How many double bonds and how many rings does it have? (c) Propose a structure for a-terpinene.arrow_forward1. Using Br2 in C2H4Br2 will result in HBr and ______. a. C2H3Cl3 b. C2H4Cl3 c. C2H2Cl3 d. none of the above 2. How many halogenation are posible in propane? a. 3 b. 8 c. 6 d. 10 3.Sulfonation of pentane will result in ________ and water. a. C5H11SO3H b. C5H12SO3H c. C5H14SO3H d. none of the above 4.Nitration of hexane will result in ________ and water. a. C6H13SO3H b. C6H15NO2 c. C6H13NO2 d. C6H14NO2 5.How many moles of O2 in heating a C12H26 (dodecane) a. 27 b. 37 c. 24 d. none of the abovearrow_forward
- Which isomer of 1-bromo-3-isopropylcyclohexane reacts faster when refluxed with potassium tert-butoxide, the cis isomer or the trans isomer? Draw the structure of the expected product from the faster-reacting compound.arrow_forwardThis triene reacts with excess maleic anhydride to produce a compound with molecular formula C14,H12,O6. Draw the structure of the product.arrow_forwardClassify the following dienes and polyenes as isolated, conjugated, cumulated, or some combination of theseclassifications.(a) cycloocta-1,4-diene (b) cycloocta-1,3-diene (c) cyclodeca-1,2-diene(d) cycloocta-1,3,5,7-tetraene (e) cyclohexa-1,3,5-triene (benzene)arrow_forward
- When free radical halogenation is performed with one of the compounds below, only a single product with the formula C5H11Cl is isolated. Which compound fits this description? Select one: a. 2,2-dimethylpropane b. cyclopentane c. pentane d. 2-methylbutanearrow_forwardChemical reactions:(1) Color KmnO4 and Cylohexane ____________________(2) Color KmnO4 and 2-methyl-Cylohexanol ____________________ (3) Color KmnO4 and Cylohexene or Limonene ____________________ (4) Color KmnO4 and Product Mixture ____________________arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
