
a)
Interpretation:
The
Concept Introduction:
The concentrations of
Consider the mixing of
Mass balance:
Charge balance:
The substitution
Assumption has to be made that
Henderson-Hasselbalch equation:
Henderson- Hasselbalch equation is rearranged form of acid dissociation expression.
For base:
Consider a basic reaction:
The Henderson- Hasselbalch equation for a basic reaction can be given as,
Where ,
The value of
The
a)
Answer to Problem 9.45P
The
Explanation of Solution
Mass balance and Charge balance is obtained if we dissolve
Mass balance =
Charge balance =
If,
Substituting the above expression into the mass balance gives,
Assume that
Then, the
If the given below values are not assumed, then equations (1) and (2) can be used
The solution is basic and
Where x=
The
b)
Interpretation:
The goal seek has to be done for the calculations done in part A.
b)
Answer to Problem 9.45P
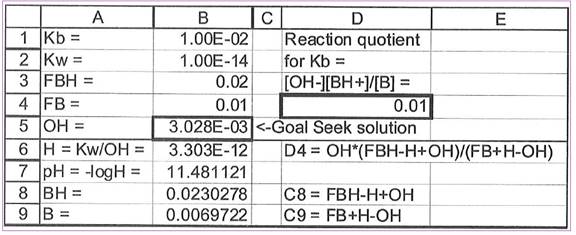
Figure 1
Explanation of Solution
The goal seek is used to vary cell
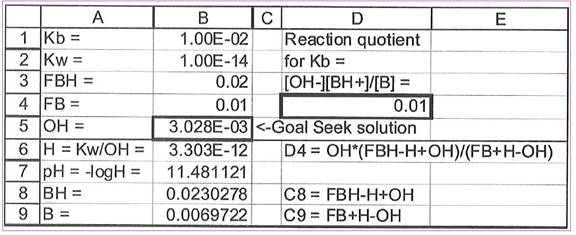
Figure 1
Want to see more full solutions like this?
Chapter 9 Solutions
Quantitative Chemical Analysis
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





