
Draw the organic product(s) formed when
a.
b.
c.
(a)
Interpretation: The organic product(s) formed by the treatment of
Concept introduction: Alcohols undergo dehydration reaction in the presence of strong acids like
Answer to Problem 9.46P
The organic product(s) formed by the treatment of
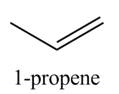
Explanation of Solution
The given reagent is
Alcohols undergo dehydration reaction in the presence of strong acids like
The organic product(s) formed by the treatment of
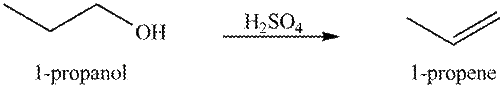
Figure 1
The organic product(s) formed by the treatment of
(b)
Interpretation: The organic product(s) formed by the treatment of
Concept introduction: An alkoxide salt is required to prepare ether. The alkoxide salts are prepared from alcohols through the Bronsted-Lowry acid-base reaction. In this reaction,
Answer to Problem 9.46P
The organic product(s) formed by the treatment of
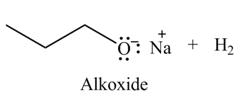 .
.
Explanation of Solution
The given reagent is
An alkoxide salt is required to prepare ether. The alkoxide salts are prepared from alcohols through the Bronsted-Lowry acid-base reaction. In this reaction,
The organic product(s) formed by the treatment of
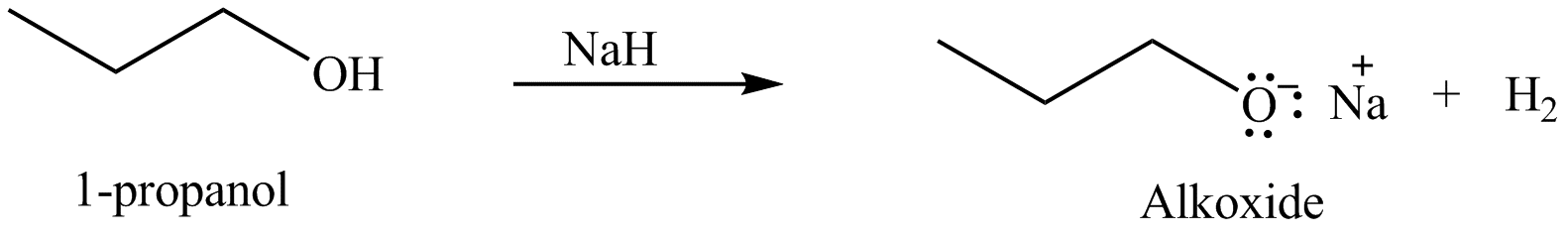
Figure 2
The organic product(s) formed by the treatment of
(c)
Interpretation: The organic product(s) formed by the treatment of
Concept introduction: The reactivity of
Answer to Problem 9.46P
The organic product(s) formed by the treatment of
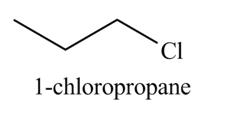
Explanation of Solution
The given reagent is
The reactivity of
The organic product(s) formed by the treatment of
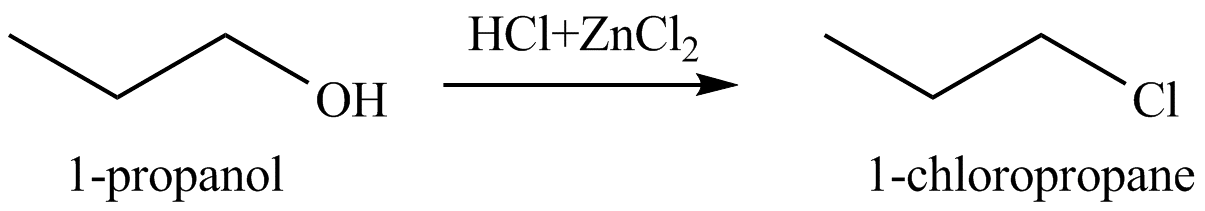
Figure 3
The organic product(s) formed by the treatment of
(d)
Interpretation: The organic product(s) formed by the treatment of
Concept introduction: The reaction of alcohols with halogen acids
Answer to Problem 9.46P
The organic product(s) formed by the treatment of
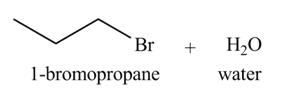
Explanation of Solution
The given reagent is
The reaction of alcohols with halogen acids
The organic product(s) formed by the treatment of
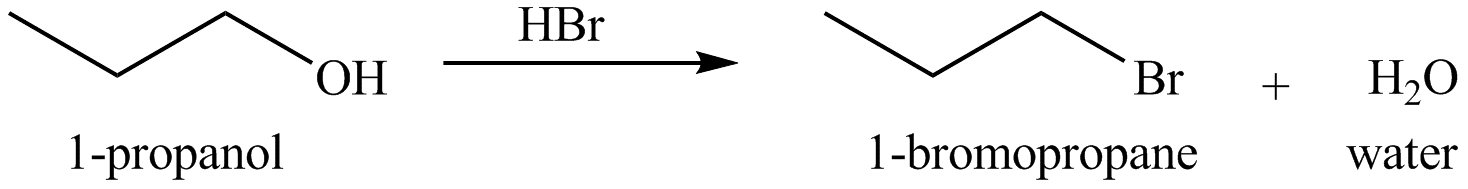
Figure 4
The organic product(s) formed by the treatment of
(e)
Interpretation: The organic product(s) formed by the treatment of
Concept introduction: Alkyl chlorides are obtained by the reaction of
Answer to Problem 9.46P
The organic product(s) formed by the treatment of
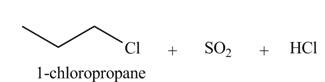
Explanation of Solution
The given reagent is
Alkyl chlorides are obtained by the reaction of
The organic product(s) formed by the treatment of
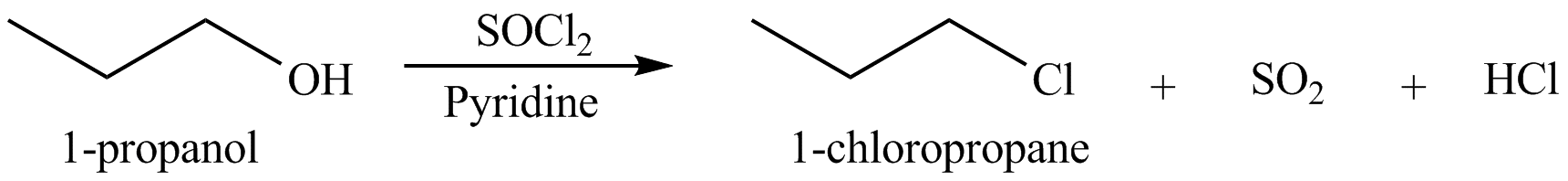
Figure 5
The organic product(s) formed by the treatment of
(f)
Interpretation: The organic product(s) formed by the treatment of
Concept introduction: Alkyl bromides are obtained by the reaction of
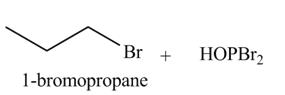
Answer to Problem 9.46P
The organic product(s) formed by the treatment of
Explanation of Solution
The given reagent is
Alkyl bromides are obtained by the reaction of
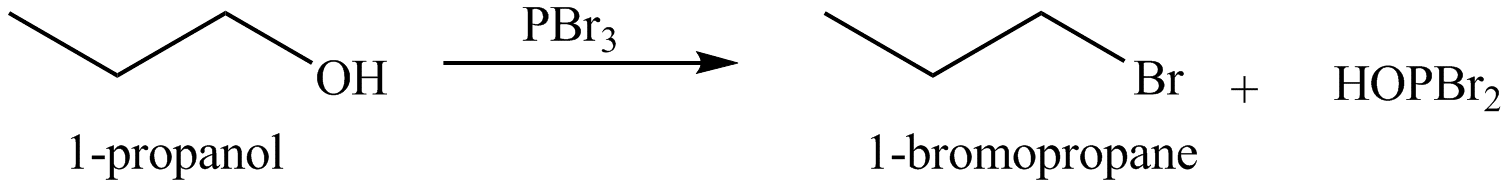
Figure 6
The organic product(s) formed by the treatment of
(g)
Interpretation: The organic product(s) formed by the treatment of
Concept introduction: Alcohols are converted into alkyl tosylates by treatment with
Answer to Problem 9.46P
The organic product(s) formed by the treatment of
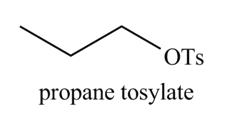
Explanation of Solution
The given reagent is
Alcohols are converted into alkyl tosylates by treatment with
The organic product(s) formed by the treatment of
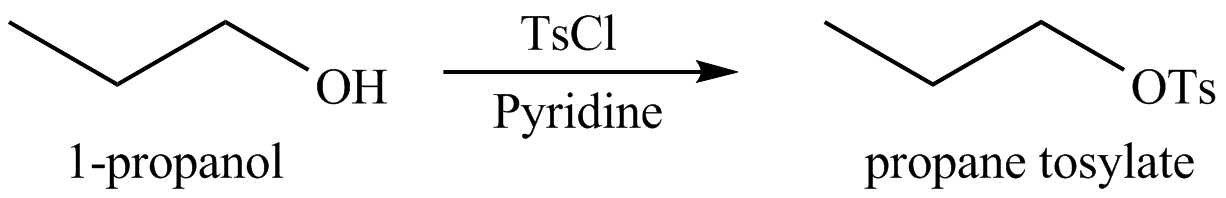
Figure 7
The organic product(s) formed by the treatment of
(h)
Interpretation: The organic product(s) formed by the treatment of
Concept introduction: An alkoxide salt is required to prepare ether. The alkoxide salts are prepared from alcohols through the Bronsted-Lowry acid-base reaction. In this reaction,
The formed alkoxide is allowed to react with an alkyl halide to obtain ether. The mechanism of the reaction is
Answer to Problem 9.46P
The organic product(s) formed by the treatment of
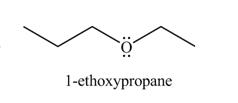
Explanation of Solution
The given reagents are
An alkoxide salt is required to prepare ether. The alkoxide salts are prepared from alcohols through the Bronsted-Lowry acid-base reaction. In this reaction,
The formed alkoxide is allowed to react with an alkyl halide to obtain ether. The mechanism of the reaction is
The organic product(s) formed by the treatment of
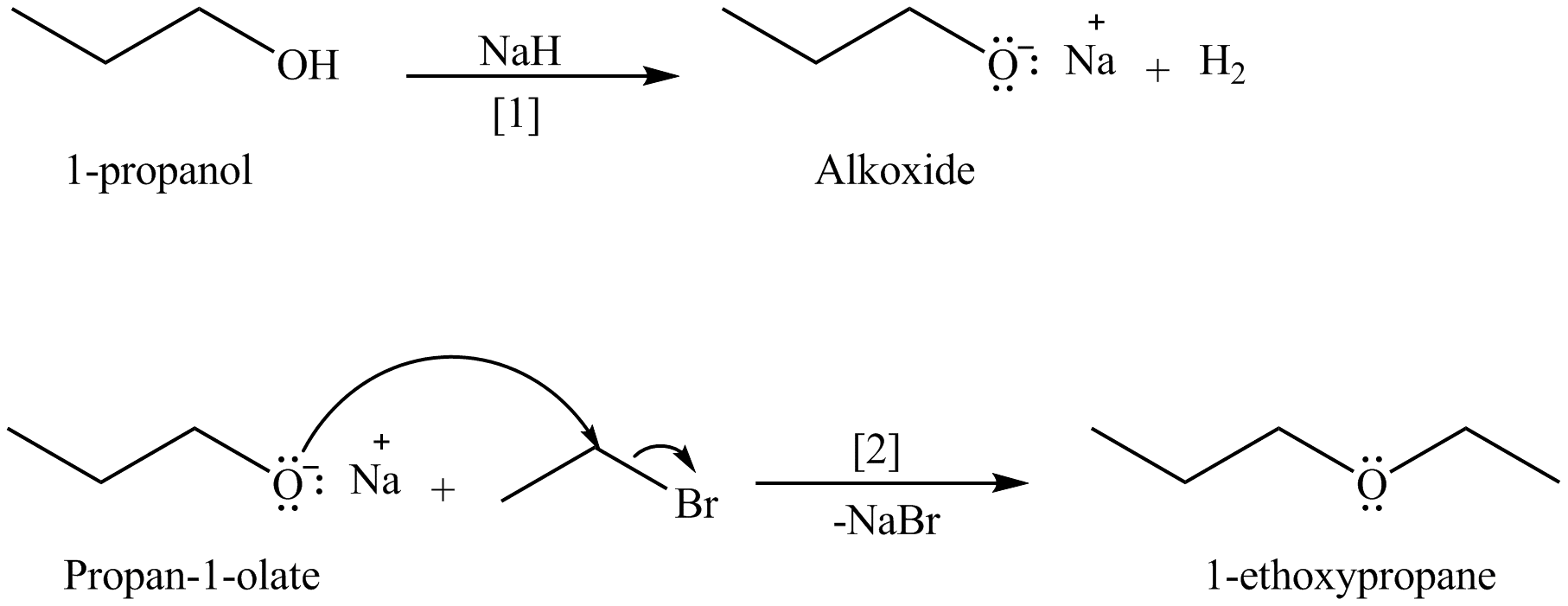
Figure 8
The organic product(s) formed by the treatment of
(i)
Interpretation: The organic product(s) formed by the treatment of
Concept introduction: Alcohols are converted into alkyl tosylates by treatment with
The formed alkyl tosylate reacts with strong nucleophile
Answer to Problem 9.46P
The organic product(s) formed by the treatment of
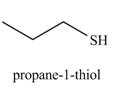
Explanation of Solution
The given reagents are
Alcohols are converted into alkyl tosylates by treatment with
The formed alkyl tosylate react with strong nucleophile
The organic product(s) formed by the treatment of
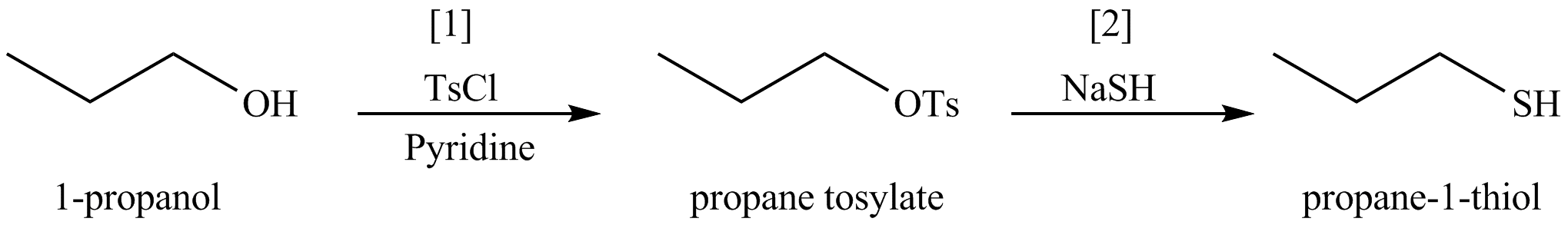
Figure 9
The organic product(s) formed by the treatment of
Want to see more full solutions like this?
Chapter 9 Solutions
Organic Chemistry -Study Guide / Solution Manual (Custom)
Additional Science Textbook Solutions
Chemistry: Atoms First
General, Organic, and Biological Chemistry: Structures of Life (5th Edition)
Introductory Chemistry (5th Edition) (Standalone Book)
The Organic Chem Lab Survival Manual: A Student's Guide to Techniques
Chemistry: The Central Science (14th Edition)
Chemistry: A Molecular Approach (4th Edition)
- a. What is the chemical structure of biphenyl, circle functional groupsdifferent than alkane, alkene, alkyne? b. Is it polar or nonpolar? _______________________ c. What is its water solubility in g/L? __________________________arrow_forward1. What functional group is produced when an aldehyde reacts with H2/Pt? A.secondary alcohol B. carboxylic acid C.hemiacetal D. primary alcohol E.alkane F.tertiary alcohol G. alkene 2. What reaction occurs when an aldehyde reacts with H2/Pt to form a primary alcohol? A. Hydration B. Hydration C. Dehydration D. Oxidation E. Reduction( hydrogentation) 3. What reaction occurs when an Ester react with H+/H2O to from a carboxylic acid and alcohol? A. Dehydration B. Reduction ( Hydrogenation) C.Hydrolysis D. Hydration E.oxidationarrow_forward___ is an example of an alkyl halide. Select one: a. KCl b. CHCl3 c. NaCl d. CF2=CF2arrow_forward
- finish the reaction by filling in any starting materials, reagents, or products as needed.arrow_forwardwhat is going on in this reaction? How do they reactant work together to produce the final product? How did the reagents work with reaction?arrow_forwardMolecule Type Boiling point (°C) CH3CH2CH3 Alkane -42 CH3CHO Aldehyde +21 CH3CH2OH Alcohol +78 i. Why is the boiling point of the aldehyde greater than that of the alkane?ii. Why is the boiling point of alcohol the highest?iii. Explain why the solubility of aldehydes and alcohols falls as the molecules get bigger.arrow_forward
- The addition reaction of an acid (HBr) to an alkene (CH3CH=CH2) follows Markovnikov's rule and involves: A) initial attack by Br– B) initial attack by Br• C) isomerization of CH3CH2CH2Br D) formation of a primary carbocation. E) formation of a secondary carbonation. (F) Formation of allyl carbocationarrow_forwardWhat alkylborane is formed from hydroboration of each alkene?arrow_forwardCan acetone react with sodium bicarbonate (NaHCO3)? Why or why not?arrow_forward
- Choose one of organic compounds (Eg: benzoic acid) that we always found in our daily life. State the source of the chosen compound and and give it's 3 benefits to us.arrow_forward6. Draw the correct structures for the following: a. what is the correct structure of 2-heptene? b. what is the correct structure of 4-octanone? c. what is the correct structure of dipropyl ether? d. what is the correct structure of 3-ethyl hexane? e. what is the correct structure of 2,2 dimethyl 1-hexanol?arrow_forwardLiAlH4 and NaBH are used to? None are correct reduce arenes. All are correct reduce carbonyl groups. reduce alkenes. Warrow_forward
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
