
Concept explainers
(a)
Interpretation:
Lewis dot symbol for
Concept Introduction:
Lewis dot symbol represents the number of valence electrons of an atom or constituent atoms bonded in a molecule. Each dot corresponds to one electron.
(a)
Explanation of Solution
Electronic configuration of
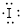
(b)
Interpretation:
Lewis dot symbol for
Concept Introduction:
Lewis dot symbol represents the number of valence electrons of an atom or constituent atoms bonded in a molecule. Each dot corresponds to one electron.
(b)
Explanation of Solution
Electronic configuration of
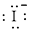
(c)
Interpretation:
Lewis dot symbol for
Concept Introduction:
Lewis dot symbol represents the number of valence electrons of an atom or constituent atoms bonded in a molecule. Each dot corresponds to one electron.
(c)
Explanation of Solution
Electronic configuration of
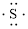
(d)
Interpretation:
Lewis dot symbol for
Concept Introduction:
Lewis dot symbol represents the number of valence electrons of an atom or constituent atoms bonded in a molecule. Each dot corresponds to one electron.
(d)
Explanation of Solution
Electronic configuration of
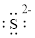
(e)
Interpretation:
Lewis dot symbol for
Concept Introduction:
Lewis dot symbol represents the number of valence electrons of an atom or constituent atoms bonded in a molecule. Each dot corresponds to one electron.
(e)
Explanation of Solution
Electronic configuration of
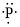
(f)
Interpretation:
Lewis dot symbol for
Concept Introduction:
Lewis dot symbol represents the number of valence electrons of an atom or constituent atoms bonded in a molecule. Each dot corresponds to one electron.
(f)
Explanation of Solution
Electronic configuration of
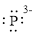
(g)
Interpretation:
Lewis dot symbol for
Concept Introduction:
Lewis dot symbol represents the number of valence electrons of an atom or constituent atoms bonded in a molecule. Each dot corresponds to one electron.
(g)
Explanation of Solution
Electronic configuration of
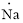
(h)
Interpretation:
Lewis dot symbol for
Concept Introduction:
Lewis dot symbol represents the number of valence electrons of an atom or constituent atoms bonded in a molecule. Each dot corresponds to one electron.
(h)
Explanation of Solution
Electronic configuration of
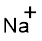
(i)
Interpretation:
Lewis dot symbol for
Concept Introduction:
Lewis dot symbol represents the number of valence electrons of an atom or constituent atoms bonded in a molecule. Each dot corresponds to one electron.
(i)
Explanation of Solution
Electronic configuration of
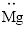
(j)
Interpretation:
Lewis dot symbol for
Concept Introduction:
Lewis dot symbol represents the number of valence electrons of an atom or constituent atoms bonded in a molecule. Each dot corresponds to one electron.
(j)
Explanation of Solution
Electronic configuration of
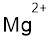
(k)
Interpretation:
Lewis dot symbol for
Concept Introduction:
Lewis dot symbol represents the number of valence electrons of an atom or constituent atoms bonded in a molecule. Each dot corresponds to one electron.
(k)
Explanation of Solution
Electronic configuration of
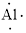
(l)
Interpretation:
Lewis dot symbol for
Concept Introduction:
Lewis dot symbol represents the number of valence electrons of an atom or constituent atoms bonded in a molecule. Each dot corresponds to one electron.
(l)
Explanation of Solution
Electronic configuration of
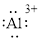
(m)
Interpretation:
Lewis dot symbol for
Concept Introduction:
Lewis dot symbol represents the number of valence electrons of an atom or constituent atoms bonded in a molecule. Each dot corresponds to one electron.
(m)
Explanation of Solution
Electronic configuration of
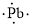
(n)
Interpretation:
Lewis dot symbol for
Concept Introduction:
Lewis dot symbol represents the number of valence electrons of an atom or constituent atoms bonded in a molecule. Each dot corresponds to one electron.
(n)
Explanation of Solution
Electronic configuration of
Want to see more full solutions like this?
Chapter 9 Solutions
General Chemistry, CHM 151/152, Marymount University
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





