
Concept explainers
(a)
Interpretation:
Lewis structure of
Concept Introduction:
Electron dot structure also known as Lewis dot structure represents the number of valence electrons of an atom or constituent atoms bonded in a molecule. Each dot corresponds to one electron.
(a)
Explanation of Solution
Total valence electrons in
Accordingly Lewis structure of
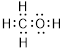
(b)
Interpretation:
Lewis structure of
Concept Introduction:
Electron dot structure also known as Lewis dot structure represents the number of valence electrons of an atom or constituent atoms bonded in a molecule. Each dot corresponds to one electron.
(b)
Explanation of Solution
Total valence electrons in
Accordingly Lewis structure of
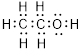
(c)
Interpretation:
Lewis structure of tetraethyllead has to be drawn.
Concept Introduction:
Electron dot structure also known as Lewis dot structure represents the number of valence electrons of an atom or constituent atoms bonded in a molecule. Each dot corresponds to one electron.
(c)
Explanation of Solution
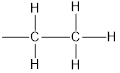
Tetraethyllead is four ethyl groups bonded to lead atom.
Structure of ethyl group is,
Tetraethyllead has molecular formula
Total valence electrons in Tetraethyllead is,
Accordingly structure of
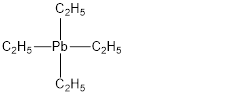
In the above structure

For simplicity the above structure is written as
(d)
Interpretation:
Lewis structure of
Concept Introduction:
Electron dot structure also known as Lewis dot structure represents the number of valence electrons of an atom or constituent atoms bonded in a molecule. Each dot corresponds to one electron.
(d)
Explanation of Solution
Total valence electrons in
Accordingly Lewis structure of
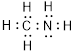
(e)
Interpretation:
Lewis structure of mustard gas
Concept Introduction:
Electron dot structure also known as Lewis dot structure represents the number of valence electrons of an atom or constituent atoms bonded in a molecule. Each dot corresponds to one electron.
(e)
Explanation of Solution
Total valence electrons in
Accordingly Lewis structure of
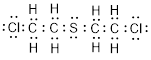
(f)
Interpretation:
Lewis structure of urea
Concept Introduction:
Electron dot structure also known as Lewis dot structure represents the number of valence electrons of an atom or constituent atoms bonded in a molecule. Each dot corresponds to one electron.
(f)
Explanation of Solution
Total valence electrons in
Accordingly Lewis structure of
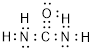
(g)
Interpretation:
Lewis structure of glycine (
Concept Introduction:
Electron dot structure also known as Lewis dot structure represents the number of valence electrons of an atom or constituent atoms bonded in a molecule. Each dot corresponds to one electron.
(g)
Explanation of Solution
Total valence electrons in
Accordingly Lewis structure of
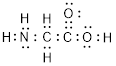
Want to see more full solutions like this?
Chapter 9 Solutions
General Chemistry, CHM 151/152, Marymount University
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





