
Concept explainers
(a)
Interpretation:
The members of the following set are to be ranked in the increasing order of ionic character. Also, the bond polarity with a polar arrow is to be indicated.
Concept introduction:
Electronegativity is the tendency of an atom to attract the shared electrons in the bond towards itself. The more electronegative atom will more attract the bonding electrons towards itself than the less electronegative atom. Therefore the electrons will spend more time with the more electronegative atom than an electropositive atom. The electronegative atom will acquire the partial negative charge and the electropositive atom will acquire a partial positive charge. The polarity is represented by an arrow towards the more electronegative element.
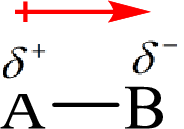
Here, B is the electronegative atom and A is the electropositive atom.
Bond polarity and ionic character can be estimated by
Here, B is the electronegative atom and A is the electropositive atom.
(a)
Answer to Problem 9.66P
The increasing order of bond polarity and ionic character is as follows:

Explanation of Solution
The formula to calculate
Substitute
The formula to calculate
Substitute
The formula to calculate
Substitute
Bond polarity and ionic character are directly related to the electronegativity difference. The increasing order of bond polarity and ionic character is as follows:

(b)
Interpretation:
The members of the following set are to be ranked in the increasing order of ionic character. Also, the bond polarity with a polar arrow is to be indicated.
Concept introduction:
Electronegativity is the tendency of an atom to attract the shared electrons in the bond towards itself. The more electronegative atom will more attract the bonding electrons towards itself than the less electronegative atom. Therefore the electrons will spend more time with the more electronegative atom than an electropositive atom. The electronegative atom will acquire the partial negative charge and the electropositive atom will acquire a partial positive charge. The polarity is represented by an arrow towards the more electronegative element.
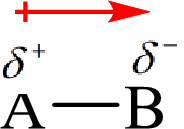
Here, B is the electronegative atom and A is the electropositive atom.
Bond polarity and ionic character can be estimated by
Here, B is the electronegative atom and A is the electropositive atom.
(b)
Answer to Problem 9.66P
The increasing order of bond polarity and ionic character is as follows:

Explanation of Solution
The formula to calculate
Substitute
The formula to calculate
Substitute
The formula to calculate
Substitute
Bond polarity and ionic character are directly related to the electronegativity difference. The increasing order of bond polarity and ionic character is as follows:

(c)
Interpretation:
The members of the following set are to be ranked in the increasing order of ionic character. Also, the bond polarity with a polar arrow is to be indicated.
Concept introduction:
Electronegativity is the tendency of an atom to attract the shared electrons in the bond towards itself. The more electronegative atom will more attract the bonding electrons towards itself than the less electronegative atom. Therefore the electrons will spend more time with the more electronegative atom than an electropositive atom. The electronegative atom will acquire the partial negative charge and the electropositive atom will acquire a partial positive charge. The polarity is represented by an arrow towards the more electronegative element.
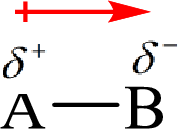
Here, B is the electronegative atom and A is the electropositive atom.
Bond polarity and ionic character can be estimated by
Here, B is the electronegative atom and A is the electropositive atom.
(c)
Answer to Problem 9.66P
The increasing order of bond polarity and ionic character is as follows:

Explanation of Solution
The formula to calculate
Substitute
The formula to calculate
Substitute
The formula to calculate
Substitute
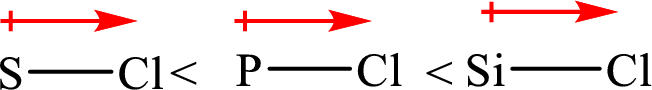
Bond polarity and ionic character are directly related to the electronegativity difference. The increasing order of bond polarity and ionic character is as follows:
Want to see more full solutions like this?
Chapter 9 Solutions
CHEM 211: CHEMISTRY VOL. 1
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





