
To find:
The enthalpy changes (
Answer to Problem 9.91QA
Solution:
a)
b)
c)
Explanation of Solution
1) Concept:
For
2) Formula:
Positive values of
3) Given:
The required average bond energy values are taken from Table A4.1 of Appendix 4.
| Bond | Bond energy (kJ/mol) |
| H-H | |
| N-H | |
| N-N | |
| N=N | |
| N=O |
4) Calculations:
a) The given balanced reaction is
The reaction involves the formation of two moles of ammonia from its constituent elements in their standard states.
First, we have to draw the Lewis structures of reactants and products into the balanced chemical equation to get the number of bonds involved in each reactant and product.
![]() +
+ ![]() ⤍
⤍ ![]()
We can construct a table using the appropriate bond energies:
| Bonds | Number of bonds(mol) | Bond energy kJ/mol | ||
| Bonds broken | ||||
| Bonds broken | ||||
| Bonds formed |
We can get the value of
b) The given balanced reaction is
The reaction involves the formation of one mole of hydrazine from its constituent elements in their standard states.
First, we have to draw the Lewis structures of reactants and products into the balanced chemical equation to get the number of bonds involved in each reactant and product.
![]() +
+ ![]() ⤍
⤍ 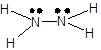
We can construct a table using the appropriate bond energies:
| Bonds | Number of bonds(mol) | Bond energy (kJ/mol) | ||
| Bonds broken | ||||
| Bonds broken | H-H | |||
| Bonds formed | N-N | |||
| Bonds formed | N-H |
We can get the value of
c)The given balanced reaction is:
The reaction involves the formation of two moles of nitrous oxide gas from its constituent elements in their standard states.
First, we have to draw the Lewis structures of reactants and products into the balanced chemical equation to get the number of bonds involved in each reactant and product.
![]() +
+ ![]() ⤍
⤍ ![]()
We can construct a table using the appropriate bond energies:
| Bonds | Number of bonds(mol) | Bond energy (kJ/mol) | ∆H | |
| Bonds broken | 2 | 945 | ||
| Bonds broken | 1 | 498 | ||
| Bonds formed | N=N | 2 | 418 | |
| Bonds formed | N=O | 2 | 607 |
We can get the value of
Conclusion:
The standard enthalpy change of a reaction can be calculated using the standard average bond energies per mole for each reactant and product.
Want to see more full solutions like this?
Chapter 9 Solutions
Chemistry: An Atoms-Focused Approach
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





