
Concept explainers
(a)
Interpretation:
The orbital energy level diagram and the number of unpaired electrons in
(a)
Answer to Problem 9D.4E
There is no unpaired electron in
Explanation of Solution
The oxidation number of zinc in
The orbital energy level diagram for
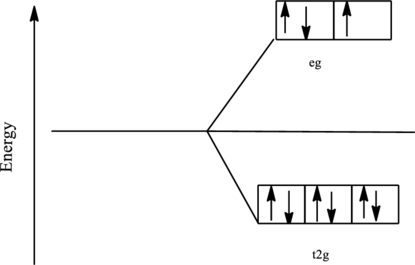
Since, water acts as a strong ligand in
(b)
Interpretation:
The orbital energy level diagram and the number of unpaired electrons in
(b)
Explanation of Solution
The oxidation number of cobalt in
The orbital energy level diagram for
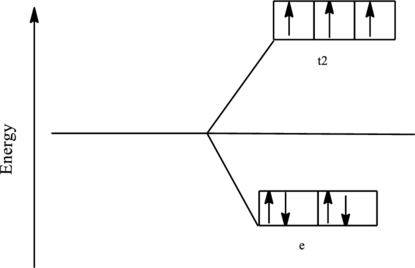
Since, chloride ion is a weak ligand the electrons are filled according to the Hund’s rule in the d-orbitals and the number of unpaired electron in cobalt complex is three.
(c)
Interpretation:
The orbital energy level diagram and the number of unpaired electrons in
(c)
Explanation of Solution
The oxidation number of iron in
The orbital energy level diagram for
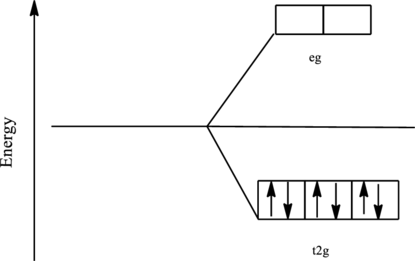
Since, cyanide acts as a strong ligand in
(d)
Interpretation:
The orbital energy level diagram and the number of unpaired electrons in
(d)
Explanation of Solution
The oxidation number of iron in
The orbital energy level diagram for
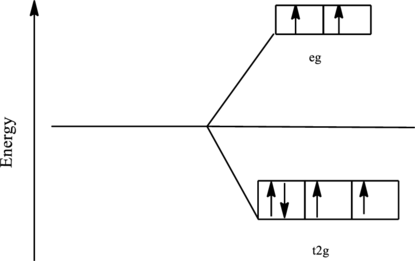
Since, fluoride ion is a weak ligand the electrons are filled according to the Hund’s rule in the d-orbitals and the number of unpaired electron in cobalt complex is four.
Want to see more full solutions like this?
Chapter 9 Solutions
CHEMICAL PRINCIPLES PKG W/SAPLING
- Four different octahedral chromium coordination compounds exist that all have the same oxidation state for chromium and have H2O and Cl as the ligands and counterions. When 1 mole of each of the four compounds is dissolved in water, how many moles of silver chloride will precipitate upon addition of excess AgNO3?arrow_forwardGiven the colors observed for VO43- (orthovanadate ion), CrO42- (chromate ion), and MnO4- (permanganate ion), what can you say about how the energy separation between the ligand orbitals and the empty d orbitals changes as a function of the oxidation state of the transition metal at the center of the tetrahedral anion?arrow_forwardExplain the differences between weak-field and strong-field metal complexes?arrow_forward
- What is the oxidation state of the metal centre in the [Cr(CO3)3(C6H6)] complex?arrow_forwardWrite the formula and the name of each complex ion- a complexion with Co3+ as the central ion and three NH3 molecules and three CN-ions as ligands, a complexion with Cr3+ as the central ion and a coordination number of 6 with ethylenediamine ligands?arrow_forwardCompare the ligand field strengths of CO and Cl, in terms of the types of bonds in which they can be involved with metal ionsarrow_forward
- How many bonds could each of the following chelating ligands form with a metal ion? acetylacetone (acacH), a common ligand in organometallic catalysts:________ bonds diethylenetriamine, used in a variety of industrial processes: ________ bonds salen, a common ligand for chiral organometallic catalysts: ________ bonds porphine, often used in supermolecular chemistry as well as catalysis; biologically, porphine is the basis for many different types of porphyrin-containing proteins, including heme proteins: _________bondsarrow_forward[CoF6]3- absorbs 700 nm (red) radiation whereas [Co(H2O)6]3+ absorbs 600 nm (orange) radiation. Please draw the crystal field splitting of the above two complexes. Clearly label the orbitals and show the electron occupation.arrow_forwardHow does Ligand Field Theory explain the interaction between the ligand with the central metal leading to increase or decrease of Δo ? Use the concepts of π-donor and π-acceptor ligands.arrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning





