
Concept explainers
The total exergy destruction for each process of an ideal dual cycle
Answer to Problem 145P
The total exergy destruction in an ideal dual cycle is
Explanation of Solution
Draw the ideal dual cycle on
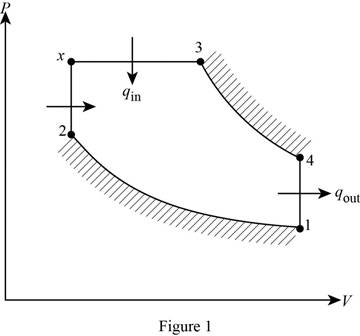
Consider, the pressure is
Write the expression of temperature and volume relation for the isentropic compression process 1-2.
Here, compression ratio is r and specific heat ratio is k.
Write the expression of pressure, and volume relation for the isentropic expansion process 1-2.
Write the expression of pressure ratio relation.
Here, pressure ratio is
Write the expression of temperature, and pressure relation for the constant volume heat addition process
Write the expression of temperature, and volume relation for the constant pressure heat addition process
Here, cutoff ratio is
Write the expression of temperature, and volume relation for the constant pressure heat addition process 3-4.
Write the expression to calculate the heat added to the cycle during process
Write the expression to calculate the heat added to the cycle during process
Here, heat input to the process
Write the expression to calculate the heat added to the cycle during process
Here, specific heat at constant pressure is
Write the expression of net heat addition to the cycle
Write the expression for exergy destruction during the process of the cycle.
Here, temperature of the surroundings is
Write the expression of entropy change for the process
Here, specific heat of air at constant volume is
Write the expression for the exergy loss for the isothermal process
Write the expression of entropy change for the isothermal process
Write the expression for the exergy loss for the process
Write the expression of entropy change for the process
Write the expression for the exergy loss for the process
Here, temperature of the sink is
Write the expression to calculate the total in an ideal dual cycle.
Conclusion:
From Table A-1E, “Molar mass, gas constant, and critical-point properties”, obtain the following properties of air at room temperature.
From Table A-2Ea, “Ideal-gas specific heats of various common gases”, obtain the value for gas content
Substitute
Substitute
Substitute
Substitute
Substitute
Substitute
Substitute
Substitute
Substitute
Substitute
Equation (VII).
The exergy loss for the isothermal process 1-2
Here
Substitute
Substitute
Substitute
Substitute
The exergy loss for the isothermal process 3-4
Here,
Substitute
Substitute
During heat rejection process the largest exergy destruction in an ideal dual cycle occur.
Substitute
Thus, the total exergy destruction in an ideal dual cycle is
Want to see more full solutions like this?
Chapter 9 Solutions
Thermodynamics: An Engineering Approach
- An Otto cycle operating in an air standard conditions has a peak temperature of 800C. If the heat added in each cycle is equal to 350kJ/kg, determine the compression ratio of the cycle.a. 5.4004 b. 5.4005 c. 5.4006 d. 5.4007arrow_forwardIn an ideal Brayton cycle with regeneration, air is compressed from 80 kPa and 10°C to 400 kPa and 175°C, is heated to 450°C in the regenerator, and is then further heated to 1000°C before entering the turbine. Under cold-air-standard conditions, the effectiveness of the regenerator is (a) 33 percent (b) 44 percent (c) 62 percent (d) 77 percent (e) 89 percentarrow_forwardAir is used as the working fluid in a simple ideal Brayton cycle that has a pressure ratio of 12, a compressor inlet temperature of 300 K, and a turbine inlet temperature of 1000 K. Determine the required mass flow rate of air for a net power output of 70 MW, assuming both the compressor and the turbine have an isentropic efficiency of 85 percent. Assume constant specific heats at room temperature.arrow_forward
- Helium is used as the working fluid in a Brayton cycle with regeneration. The pressure ratio of the cycle is 8, the compressor inlet temperature is 300 K, and the turbine inlet temperature is 1800 K. The effectiveness of the regenerator is 75 percent. Determine the thermal efficiency and the required mass flow rate of helium for a net power output of 60 MW, assuming both the compressor and the turbine have an isentropic efficiency of (a) 100 percent and (b) 80 percent.arrow_forwardA Brayton cycle with regeneration using air as the working fluid has a pressure ratio of 7. The minimum and maximum temperatures in the cycle are 310 and 1150 K. Assuming an isentropic efficiency of 75 percent for the compressor and 82 percent for the turbine and an effectiveness of 65 percent for the regenerator, determine the air temperature at the turbine exit.arrow_forwardAn ideal dual cycle has a compression ratio of 14. At the start of the compression process, air is at 100 kPa and 300 K, and at 2200 K at the start of the heat addition phase. Heat transfer to air occurs in two stages, one at constant volume and one at constant pressure, and totals 1520.4 kl/kg. Determine the temperature after the isentropic expansion process, the proportion of heat transferred at constant volume, and t thermal efficiency of the cycle using changing specific heats for airarrow_forward
- Helium is used as the working fluid in a Brayton cycle with regeneration. The pressure ratio of the cycle is 8, the compressor inlet temperature is 300 K, and the turbine inlet temperature is 1800 K. The effectiveness of the regenerator is 75 percent. Determine the thermal efficiency and the required mass flow rate of helium for a net power output of 60 MW, assuming both the compressor and the turbine have an isentropic efficiency of 80 percent.arrow_forwardConsider a regenerative Brayton cycle operating with a pressure ratio of 8, where the air enters the compressor at 300 K and 100 kPa, while it enters the turbine at 1060 K. Assume that both the compressor and turbine are isentropic, that specific heats vary with temperature, and that the regenerator has an effectiveness of 74%. Round all intermediate calculations to three decimal places. 1. How much net work is produced in kJ/kg? Round your answer to two decimal places. 2. What is the thermal efficiency as a percentage (0% - 100%)? Round your answer to one decimal place. 3. What is the thermal efficiency as a percentage (0% - 100%) if there was no regenerator? Round your answer to one decimal place.arrow_forwardAn Otto cycle operating in an air standard conditions has a peak temperature of 8000C. If the heat added in each cycle is equal to 350kJ/kg, determine the compression ratio of the cycle. a. 5.4004 b. 5.4005 c. 5.4006 d. 5.4007arrow_forward
- An Otto cycle with air as the working fluid has a compression ratio of 10.4. Under cold-air-standard conditions, the thermal efficiency of this cycle is (a) 10 percent (b) 39 percent (c) 61 percent (d) 79 percent (e) 82 percentarrow_forwardOtto cycle operating under air standard condition has a compression ratio of 8.0. If the heat added on the cycle amounts to 3500kJ/kg of working substance, determine the peak temperature of the cycle.a. 899.6239C b. 899.6237C c. 899.6287C d. 899.6537Carrow_forwardIf the isentropic compression ratio of a Carnot cycle is 5 and the lower temperature limit of the cycle is 25C, determine the power that the engine can produce if the heat added amounts to 45kW. Assume that the cycle is an air standard cycle.a. 21.3609kW b. 21.3610kW c. 21.3611kW d. 21.3612kWarrow_forward
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY





