
The exergy destruction for each of the processes of the cycle
Answer to Problem 147P
The exergy destruction associated with process 1-2 is
The exergy destruction associated with process 5-3 is
The exergy destruction associated with process 3-4 is
The exergy destruction associated with process 6-1 is
The exergy destruction associated at regenerator is
Explanation of Solution
Draw the
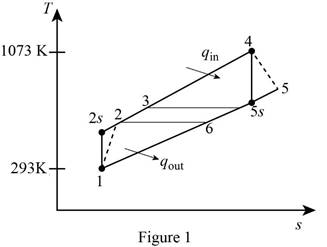
Write the expression for the temperature and pressure relation for the isentropic process 1-2.
Here, the pressure ratio is
Write the expression for the efficiency of the compressor
Here, the specific heat at constant pressure is
Write the expression for the temperature and pressure relation ratio for the expansion process 4-5s.
Write the expression for the efficiency of the turbine
Apply first law to the heat exchanger.
Write the expression for the net work done per kg for the Brayton cycle with regeneration
Here, the specific heat at constant pressure is
Write the expression of heat addition to the regenerative Brayton cycle
Write the expression for rate of heat rejection in the regenerative Brayton cycle
Write the expression for the exergy destruction during the process of as steam from an inlet to exit state.
Write the expression of exergy destruction for process 1-2
Here, pressure at state 2 is
Write the expression of exergy destruction for process 5-3
Here, pressure at state 5 is
Write the expression of exergy destruction for process 3-4
Here, pressure at state 7 is
Write the expression of exergy destruction for process 6-1
Here, pressure at state 10 is
Write the expression of exergy destruction for regenerator
Conclusion:
Substitute
Substitute
Substitute
Substitute
The cold airstream
Substitute
Substitute
Substitute
Substitute
Substitute
Substitute
Thus, the exergy destruction associated with process 1-2 is
Here,
Substitute
Thus, the exergy destruction associated with process 5-3 is
Substitute
Thus, the exergy destruction associated with process 3-4 is
Here,
Substitute
Thus, the exergy destruction associated with process 6-1 is
Substitute
Thus, the exergy destruction associated at regenerator is
Want to see more full solutions like this?
Chapter 9 Solutions
Thermodynamics: An Engineering Approach
- Air is used as the working fluid in a simple ideal Brayton cycle that has a pressure ratio of 12, a compressor inlet temperature of 300 K, and a turbine inlet temperature of 1000 K. Determine the required mass flow rate of air for a net power output of 70 MW, assuming both the compressor and the turbine have an isentropic efficiency of 85 percent. Assume constant specific heats at room temperature.arrow_forwardAir enters the compressor of a gas turbine at 100 kPa and 25°C. Determine the back work rate and thermal efficiency of the Brayton cycle for a pressure ratio of 5 and a maximum temperature of 850°C.arrow_forwardIn an air standard gas turbine cycle, air at 14.5 psia and 70 F is first compressed to 80 psia in a compressor of 82 percent efficiency. The hot air leaving the combustion chamber at 1250 F is expanded back to 14.5 psia in a turbine of 85 percent efficiency. If a regenerator is inserted into the cycle to heat the air leaving the compressor to 650 F, determine the thermal efficiency of the cycle and the effectiveness of the regenerator.arrow_forward
- A simple Rankine cycle has a pump with an isentropic efficiency of 70%. The inlet and outlet pressures of the turbine are 6 MPa and 0.075 MPa, respectively, and steam enters the turbine at 550°C. Determine a) the isentropic efficiency of the turbine if the quality at the turbine outlet is to be ? = 1, b) the thermal efficiency of the cycle, c) the rate of heat input into the boiler if the net power output of the cycle is 10 MW.arrow_forwardAnswer All! Air enters the compressor of a gas turbine at 100 kPa and 300 K with a volume flow rate of 5 m3/s. The compressor pressure ratio is 10 and its isentropic efficiency is 85%. At the inlet to the turbine, the pressure is 950 kPa and the temperature is 1400 K. The turbine has an isentropic efficiency of 88% and the exit pressure is 100 kPa. On the basis of an air-standard analysis, what is the thermal efficiency of the cycle in percent?a. 42.06 c. 31.89b. b. 60.20 d. 25.15 in a gas turbine operating on the air-standard cycle, the air enters the compressor at 100 kPa and 30oC at the rate of 20 m3/s and is compressed to 500 kPa. The maximum temperature is 780oC and the exit pressure of the turbine is 100 kPa. Determine the net turbine power.a. 4853 kW c. 4483 kWb. 4358 kW d. 4538 kW A gas turbine working on air-standard Brayton cycle has air enter into the compressor at atmospheric condition and 22oC. The pressure ratio is 9 and the maximum temperature in…arrow_forwardAir enters the compressor of a regenerative gasturbine engine at 310 K and 100 kPa, where it is compressed to 900 kPa and 650 K. The regenerator has an effectiveness of 80 percent, and the air enters the turbine at 1400 K. For a turbine efficiency of 90 percent, determine the thermal efficiency. Assume variable specific heats for air.arrow_forward
- Helium is used as the working fluid in a Brayton cycle with regeneration. The pressure ratio of the cycle is 8, the compressor inlet temperature is 300 K, and the turbine inlet temperature is 1800 K. The effectiveness of the regenerator is 75 percent. Determine the thermal efficiency and the required mass flow rate of helium for a net power output of 60 MW, assuming both the compressor and the turbine have an isentropic efficiency of (a) 100 percent and (b) 80 percent.arrow_forwardThe 7FA gas turbine manufactured by General Electric is reported to have an efficiency of 35.9 percent in the simple-cycle mode and to produce 159 MW of net power. The pressure ratio is 14.7 and the turbine inlet temperature is 1288°C. The mass flow rate through the turbine is 1,536,000 kg/h. Taking the ambient conditions to be 20°C and 100 kPa, determine: (a) the isentropic efficiency of the turbine and the compressor, (b) the thermal efficiency of this gas turbine if a regenerator with an effectiveness of 80 percent is added. Answers: (a) 0.849, 0.924 (b) 0.496arrow_forwardConsider a regenerative Brayton cycle operating with a pressure ratio of 8, where the air enters the compressor at 300 K and 100 kPa, while it enters the turbine at 1060 K. Assume that both the compressor and turbine are isentropic, that specific heats vary with temperature, and that the regenerator has an effectiveness of 74%. Round all intermediate calculations to three decimal places. 1. How much net work is produced in kJ/kg? Round your answer to two decimal places. 2. What is the thermal efficiency as a percentage (0% - 100%)? Round your answer to one decimal place. 3. What is the thermal efficiency as a percentage (0% - 100%) if there was no regenerator? Round your answer to one decimal place.arrow_forward
- Helium is used as the working fluid in a Brayton cycle with regeneration. The pressure ratio of the cycle is 8, the compressor inlet temperature is 300 K, and the turbine inlet temperature is 1800 K. The effectiveness of the regenerator is 75 percent. Determine the thermal efficiency and the required mass flow rate of helium for a net power output of 60 MW, assuming both the compressor and the turbine have an isentropic efficiency of 100 percent.arrow_forwardA gas-turbine power plant operates on a simple Brayton cycle with air as the working fluid. The air enters the turbine at 120 psia and 2000 R and leaves at 15 psia and 1200 R. Heat is rejected to the surroundings at a rate of 6400 Btu/s, and air flows through the cycle at a rate of 40 lbm/s. Assuming the turbine to be isentropic and the compressor to have an isentropic efficiency of 80 percent, determine the net power output of the plant. Account for the variation of specific heats with temperature.arrow_forwardFor a specified pressure ratio, why does multistage compression with intercooling decrease the compressor work, and multistage expansion with reheating increase the turbine work?arrow_forward
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY





