
Concept explainers
Interpretation:
The structure of the compound with molecular formula
Concept introduction:
Mass spectrometry is a technique used for measuring the masses of atoms and molecules with great accuracy.
In a mass spectrometer, the vapor of organic compound is bombarded with a beam of high energy electrons that makes the neutral molecule lose an electron and converts it to a radical cation known as a molecular ion.
In
In
Nuclear Magnetic Resonance (NMR) is one of the most capable analytical techniques used for determining the functional groups and how the atoms are structured and arranged in a molecule.
Few elements, such as
In
Induced magnetic field consists of electricity generated from movement in a magnetic field.
The position of a signal on x-axis in the
The number of signals in
The area covered by the signal is proportional to the number of equivalent protons causing the signal.
The hydrogen atoms on adjacent carbon atoms split the signal into two or more peaks. One, two or three hydrogen atoms split the signal into two, three or four peaks described as doublet, triplet or quartet respectively.
A decrease in the electron density around a proton deshields the signal downfield at a larger value of chemical shift.
An increase in electron density shields the signal upfield at a lower value of chemical shift.
The peak at
The peak at
The peak at
Answer to Problem 1PP
Solution: The structure of the compound with molecular formula
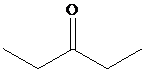
Explanation of Solution
The figure given below represents the
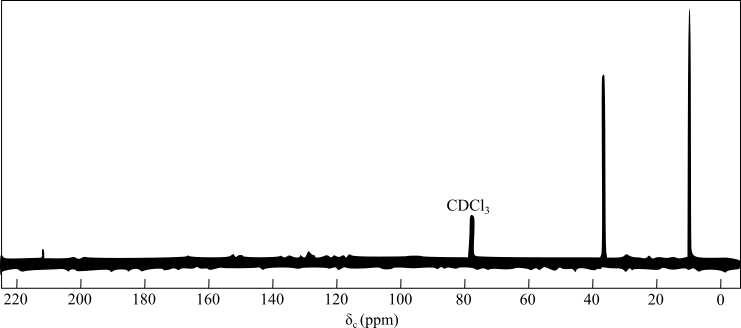
In DEPT
The peak at
The peak at
The peak at
The presence of two signals in the alkyl group region indicates the presence of symmetry in the compound and the two unique carbon atoms.
Therefore, the structure of the compound is as:

The structure of the compound with molecular formula

Want to see more full solutions like this?
Chapter A Solutions
CHEM 313:ORG.CHEM V1 W/WLYLS BLKBRD >B
- A student prepares a solution of his dye and measures its absorbance using a 1 cm cuvette. The absorbance is 0.37 and the concentration of the solution is 2.0 * 10-5 M. Determine the molar extinction coefficient at this wavelength (Integer only) answer: Which of the following transition would be spin allowed? Group of answer choices a n to p b n to s c n to s* d n to p* e p to p* Which of the following transitions will exhibit the shortest wavelength in the UV-Vis spectrum? Group of answer choices a p to s* b s to s* c n to p* d p to p* e n to s*arrow_forwardWhich of the following relationships between absorbance and %Transmittance are incorrect? correct incorrect A = 2 - log10%T. correct incorrect A = log101000/%T. correct incorrect A = 1 - log10 1/%T.arrow_forwardP-Chem question: Assume there are two pairs of harmonic oscillatorcs A-A and B-B. If A and B are isotopes, given mB=3mA, a.Express the ratio of their vibrational frequencies in terms of mA b.Given force constant of A-A, kf, express the absorption wavelength between adjacent energy levelsarrow_forward
- Project 2: Food Dye Spectroscopy What method will you use to assess the absorbance-concentration relationship? (The most obvious method is to see if there is a linear relationship between the absorbance and the concentration. What methods do you know that might reveal such a linear relationship?)arrow_forward3. Find the Structural Formula in 1H-NMR Spectra of C3H7Br. Compute the IHD, integration, identify the species per peak, and solve the structural formula. A. Computation B. Analysis/ Interpretation: 1. How many types of H(signals)? 2. What types of H(chemical shift)? 3. How many H of each type are there(integration)? 4. What are the connectivity(coupling patterns)?arrow_forwardDetermine splitting for all CHx groups using n+1 rule then match to appropriate spectrum drawing CH or OH group above each of the peaks.arrow_forward
- TOPIC: ORGANIC CHEMISTRY Solve step by step please thank you (explain) The propane CH3-CH2-CH3, how many signals in 1H-NMR will it have? a) 1 b) 2 c) 3 d) 4arrow_forwardIn absorbance spectroscopy, the molar absorptivity coefficient: a) does not have units b) always is a constant for a given substance c) depends on the wavelength d) no correct answerarrow_forwardWhat is true about the IR spectrum of 2,3-dimethyl-2-butene? Question 36 options: The peaks at 1600-1700 cm-1 (C=C) and at 3100 cm-1 (C-H; sp2-s bond) will not be observed There will be a peak (C=C) at 1600-1700 cm-1 There will be a peak (C-H; sp2-s bond) at 3100 cm-1 There will be a peak (C-H; sp2-s bond) just below 3000 cm-1 There will be a peak (C-H; sp3-s bond) above 3000 cm-1arrow_forward
- Compare Proton and Carbon 13 NMR? Please answer at your own easy words. Answer should be to the point.arrow_forwardWhich has a lower wavenumber, C-H or C-C? C-C mass is 12+12= 24 C-H mass is 12+1= 12 , the one with the highest mass has the lowest wavenumber, so the answer, is it C-C?arrow_forwardAnswer of letter c)arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
