
Concept explainers
(a)
Interpretation:
The structure for the given IUPAC name is to be drawn.
Concept introduction:
The root name in the IUPAC name is the parent
Answer to Problem A.44P
The structure for the given IUPAC name is as follows:
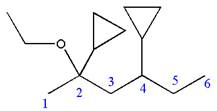
Explanation of Solution
The given IUPAC name is
The root name ‘hexane’ indicates the parent chain is of six carbon atoms.
Thus, the chain is numbered as shown below:
![]()
In the IUPAC name
Hence, the structure of
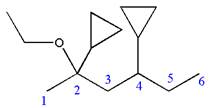
The structure for the given molecules is drawn from the root name, prefix and locator of substituent.
(b)
Interpretation:
The structure for the given IUPAC name is to be drawn.
Concept introduction:
The root name in the IUPAC name is the parent alkane. The longest continuous carbon chain must be drawn from the root name and numbered. The substituents are attached to the desired carbon number according to locator numbers given in the IUPAC name. The prefix indicates the number of identical substituents in the molecule.
Answer to Problem A.44P
The structure for
Explanation of Solution
The given IUPAC name is
The root name cyclohexane indicates the parent is a ring of six carbon atoms.
Hence, the structure of
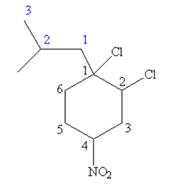
The structure for the given molecules is drawn from the root name, prefix and locator of substituent.
(c)
Interpretation:
The structure for the given IUPAC name is to be drawn.
Concept introduction:
The root name in the IUPAC name is the parent alkane. The longest continuous carbon chain must be drawn from the root name and numbered. The substituents are attached to the desired carbon number according to locator numbers given in the IUPAC name. The prefix indicates the number of identical substituents in the molecule.
Answer to Problem A.44P
The structure for the given IUPAC name is as follows:
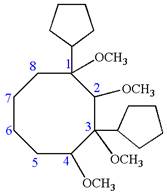
Explanation of Solution
The given IUPAC name is
The root name cyclooctane indicates the parent is a ring of eight carbon atoms.
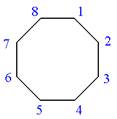
Hence, the structure of
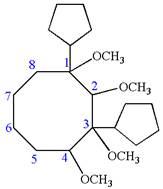
The structure for the given molecules is drawn from the root name, prefix and locator of substituent.
(d)
Interpretation:
The structure for the given IUPAC name is to be drawn.
Concept introduction:
The root name in the IUPAC name is the parent alkane. The longest continuous carbon chain must be drawn from the root name and numbered. The substituents are attached to the desired carbon number according to locator numbers given in the IUPAC name. The prefix indicates the number of identical substituents in the molecule.
Answer to Problem A.44P
The structure for the given IUPAC name is as follows:
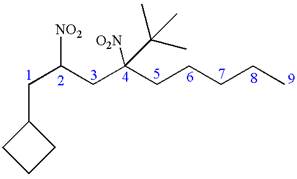
Explanation of Solution
The given IUPAC name is
The root name nonane indicates the parent is a chain of nine carbon atoms.
![]()
Hence, the structure of
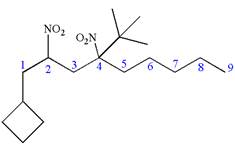
The structure for the given molecules is drawn from the root name, prefix and locator of substituent.
(e)
Interpretation:
The structure for the given IUPAC name is to be drawn.
Concept introduction:
The root name in the IUPAC name is the parent alkane. The longest continuous carbon chain must be drawn from the root name and numbered. The substituents are attached to the desired carbon number according to locator numbers given in the IUPAC name. The prefix indicates the number of identical substituents in the molecule.
Answer to Problem A.44P
The structure for the given IUPAC name is as follows:
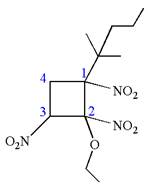
Explanation of Solution
The given IUPAC name is
The root name cyclobutane indicates the parent is a ring of four carbon atoms.
Hence, the structure of
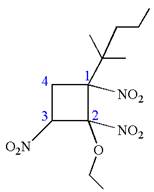
The structure for the given molecules is drawn from the root name, prefix and locator of substituents.
(f)
Interpretation:
The structure for the given IUPAC name is to be drawn.
Concept introduction:
The root name in the IUPAC name is the parent alkane. The longest continuous carbon chain must be drawn from the root name and numbered. The substituents are attached to the desired carbon number according to locator numbers given in the IUPAC name. The prefix indicates the number of identical substituents in the molecule.
Answer to Problem A.44P
The structure for the given IUPAC name is as follows:
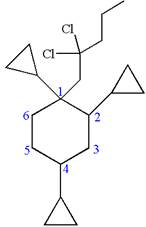
Explanation of Solution
The given IUPAC name is
The root name cyclohexane indicates the parent is a ring of six carbon atoms.
Hence, the structure of
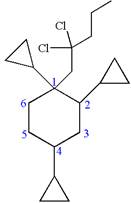
The structure for the given molecules is drawn from the root name, prefix and locator of substituent.
(g)
Interpretation:
The structure for the given IUPAC name is to be drawn.
Concept introduction:
The root name in the IUPAC name is the parent alkane. The longest continuous carbon chain must be drawn from the root name and numbered. The substituents are attached to the desired carbon number according to locator numbers given in the IUPAC name. The prefix indicates the number of identical substituents in the molecule.
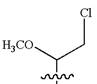
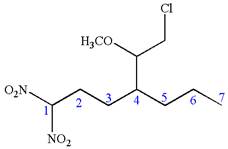
Answer to Problem A.44P
The structure for the given IUPAC name is as follows:
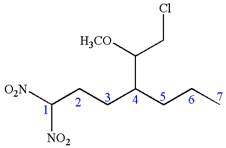
Explanation of Solution
The given IUPAC name is
The root name heptane indicates the parent is a chain of seven carbon atoms.
![]()
Hence, the structure of
The structure for the given molecules is drawn from the root name, prefix and locator of substituent.
(h)
Interpretation:
The structure for the given IUPAC name is to be drawn.
Concept introduction:
The root name in the IUPAC name is the parent alkane. The longest continuous carbon chain must be drawn from the root name and numbered. The substituents are attached to the desired carbon number according to locator numbers given in the IUPAC name. The prefix indicates the number of identical substituents in the molecule.
Answer to Problem A.44P
The structure for the given IUPAC name is as follows:
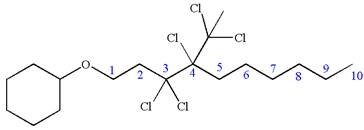
Explanation of Solution
The given IUPAC name is
The root name decane indicates the parent is a chain of ten carbon atoms.
![]()
Hence, the structure of
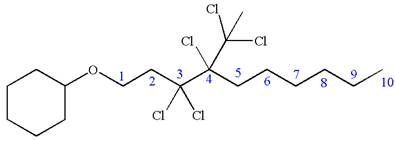
The structure for the given molecules is drawn from the root name, prefix and locator of substituent.
Want to see more full solutions like this?
Chapter A Solutions
ORGANIC CHEMISTRY E-BOOK W/SMARTWORK5
- 1,2,3,4,5,6-Hexachlorocyclohexane shows cis,trans isomerism. At one time, a crude mixture of these isomers was sold as an insecticide. The insecticidal properties of the mixture arise from one isomer, known as lindane, which is cis-1,2,4,5-trans- 3,6-hexachlorocyclohexane. Q.) Which of the alternative chair conformations of lindane is more stable? Explain.arrow_forwardA structure with the formula C7H14O must be drawn that contains one stereocenter, an alcohol, and a disubstituted alkene including what the IUPAC name is for this compound. Dashes/wedges should be used to show which enantiomer is being drawn. In addition, what is the enantiomer drawing and IUPAC name of this compound, as well as the diastereomer of this compound and IUPAC name?arrow_forwardExplain briefly and clearly the following concepts, taking as reference the molecule of n-butane and the corresponding drawings or illustrations. See pages 149-152 of the book Organic Chemistry, sixth edition (J. G. Smith). 4. What is steric hindrance in a conformation? Then draw a picture to illustrate the concept? 5. What is the torsional stress of a conformation? Then draw a picture to illustrate the concept? 6. Describe 1,3-diaxial interaction and illustrate with a specific example.arrow_forward
- According to the following structure, draw the Newmann projections in which you must indicate: a) the most stable conformation and explain why it is more stable, b) the least stable conformation and explain why; c) a conformation where there is a gauche interaction.arrow_forwardWhen bromine is added to two beakers, one containing phenyl isopropyl ether and the other containing cyclohexene, the bromine color in both beakers disappears. What observation could you make while performing this test that would allow you to distinguish the alkene from the aryl ether?arrow_forwardProvide the correct IUPAC name for the skeletal (line-bond) structure shown here. Stereochemistry is ignored.arrow_forward
- Which do you expect to be the more stable conformation of cis-1,3-dimethylcyclobutane, A or B? Why?arrow_forwarda) When (Z)-3-methylhex-3-ene undergoes hydroboration–oxidation, two isomeric products are formed. Give their structures, and label each asymmetric carbon atom as (R) or (S). What is the relationship between these isomers?arrow_forwardDraw the structures based on iupac name digitally (highly suggest chem-space .com for easy drawing) 1,1-Dibromo-4-isopropylcyclohexane 4-sec-Butyl-2-chlorononane 1.1-Dibromo-4-Pert-Butylcyclohexanearrow_forward
- draw and name two structures that match the description of a trans-dihalocyclopentanearrow_forwardGive the structure corresponding to following name. cis-1,3-dichlorocyclopentanearrow_forwardFor the following compound - calculate the degrees of unsaturation and enter that number into the space provided.show the completed mathematical calculation. Figures form Academia Obscura (anthonycrasto.wordpress.com)arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
