
Concept explainers
(a)
Interpretation:
The structure for the given molecule is to be drawn.
Concept introduction:
When assigning priorities to substituents, the atom having the greater
Answer to Problem C.32P
The structure of
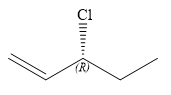
Explanation of Solution
The given name is:
In this compound, the root is pentene. The compound contains a double bond between C1 and C2 carbon atoms. The longest continuous carbon chain contains five carbon atoms. A chlorine is attached to C3 carbon atom of the root. The structure of the molecule without considering its stereochemistry is:
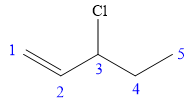
There is one chiral carbon at C3 in the above molecule. The required configuration at the chiral carbon atom is R.
At C3 carbon atom, the four substituents are:
Applying the first tiebreaker,
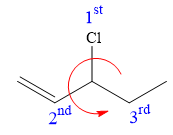
Thus, in order to have R configuration at C3 carbon atom, the first-priority substituent must be attached by a dash bond. Therefore, the correct structure for

The structure for the given name is drawn as shown above.
(b)
Interpretation:
The structure for the given molecule is to be drawn.
Concept introduction:
When assigning priorities to substituents, the atom having the greater atomic number has higher priority. In case of comparison between isotopes, the one having the greater atomic mass gets higher priority. When the fourth priority substituent is pointing away (it is attached by a dash bond) and the first, second, and third priority substituents are arranged clockwise, the configuration is R. When the fourth priority substituent is pointing away (it is attached by a dash bond) and the first, second, and third priority substituents are arranged counterclockwise, the configuration is S. If the fourth priority substituent is attached by a wedge bond, then the clockwise or counterclockwise arrangement of the first, second, and third priority substituents is determined and that arrangement is reversed before assigning R or S. If the fourth priority substituent is in the plane of the page, then it is switched with the substituent that points away. Then the clockwise or counterclockwise arrangement of the first, second and third priority substituents is determined and that arrangement is reversed before assigning R or S. When writing the IUPAC name, the R or S designation is written in parenthesis for each asymmetric carbon atom and hyphens are used to separate those designations from the rest of the IUPAC name.
Answer to Problem C.32P
The structure of
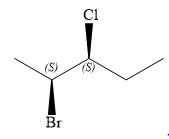
Explanation of Solution
The given name is
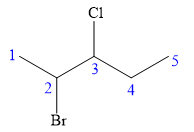
There are two chiral carbon atoms at C2 and C3 in the above molecule. The required configuration at both the chiral carbon atoms is S.
At C2 carbon atom, the four substituents are:
Applying the first tiebreaker,
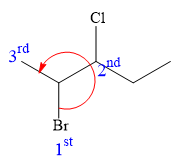
Thus, in order to maintain this arrangement of substituents at the C2 carbon atom, the fourth-priority substituent must be attached by a dash bond
At C3 carbon atom, the four substituents are:
Applying the first tiebreaker,
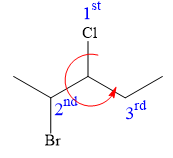
Thus, in order to maintain this arrangement of substituents at the C3 carbon atom, the fourth-priority substituent must be attached by a dash bond
Therefore, the correct structure for

The structure for the given name is drawn as shown above.
(c)
Interpretation:
The structure for the given molecule is to be drawn.
Concept introduction:
When assigning priorities to substituents, the atom having the greater atomic number has higher priority. In case of comparison between isotopes, the one having the greater atomic mass gets higher priority.
When the fourth priority substituent is pointing away (it is attached by a dash bond) and the first, second, and third priority substituents are arranged clockwise, the configuration is R.
When the fourth priority substituent is pointing away (it is attached by a dash bond) and the first, second, and third priority substituents are arranged counterclockwise, the configuration is S.
If the fourth priority substituent is attached by a wedge bond, then the clockwise or counterclockwise arrangement of the first, second, and third priority substituents is determined and that arrangement is reversed before assigning R or S.
If the fourth priority substituent is in the plane of the page, then it is switched with the substituent that points away. Then the clockwise or counterclockwise arrangement of the first, second and third priority substituents is determined and that arrangement is reversed before assigning R or S. When writing the IUPAC name, the R or S designation is written in parenthesis for each asymmetric carbon atom and hyphens are used to separate those designations from the rest of the IUPAC name.
Answer to Problem C.32P
The structure of
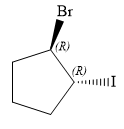
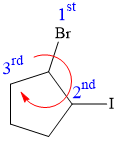
Explanation of Solution
The given name is:
In this compound, the root is cyclopentane. One bromine atom and one iodine atom is attached to C1 and C2 carbon atom of the root.
The structure of the molecule, without considering its stereochemistry, is:
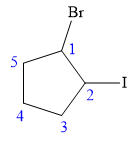
There are two chiral carbon atoms at C1 and C2 in the above molecule. The required configuration at both the chiral carbon atoms is R.
At C1 carbon atom, the four substituents are:
Applying the first tiebreaker,
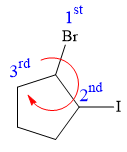
Thus, in order to maintain this arrangement of substituents at C2 carbon atom, the fourth-priority substituent must be attached by a dash bond
At C3 carbon atom, the four substituents are:
Applying the first tiebreaker,

Thus, in order to maintain this arrangement of substituents at the C3 carbon atom, the fourth-priority substituent must be attached by a dash bond
Therefore, the correct structure for
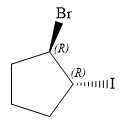
The structure for the given name is drawn as shown above.
(d)
Interpretation:
The structure for the given molecule is to be drawn.
Concept introduction:
When assigning priorities to substituents, the atom having the greater atomic number has higher priority. In case of comparison between isotopes, the one having the greater atomic mass gets higher priority. When the fourth priority substituent is pointing away (it is attached by a dash bond) and the first, second, and third priority substituents are arranged clockwise, the configuration is R. When the fourth priority substituent is pointing away (it is attached by a dash bond) and the first, second, and third priority substituents are arranged counterclockwise, the configuration is S. If the fourth priority substituent is attached by a wedge bond, then the clockwise or counterclockwise arrangement of the first, second, and third priority substituents is determined and that arrangement is reversed before assigning R or S. If the fourth priority substituent is in the plane of the page, then it is switched with the substituent that points away. Then the clockwise or counterclockwise arrangement of the first, second, and third priority substituents is determined and that arrangement is reversed before assigning R or S. When writing the IUPAC name, the R or S designation is written in parenthesis for each asymmetric carbon atom and hyphens are used to separate those designations from the rest of the IUPAC name.
Answer to Problem C.32P
The structure of
Explanation of Solution
The given name is:
In this compound, the root is cyclohexene. The cyclohexane ring contains one double bond between C1 and C2 carbon atoms. One chlorine atom is attached at the C3 carbon atom of the root. The structure of the molecule without considering its stereochemistry is:
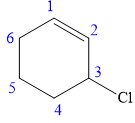
There is one chiral carbon at C3 in the above molecule. The required configuration at the chiral carbon atom is S.
At C3 carbon atom, the four substituents are:
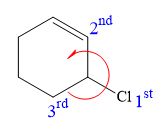
Thus, in order to maintain this arrangement of substituents at the C2 carbon atom, the first priority substituent should be attached by a dash bond. Therefore, the correct structure for
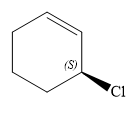
The structure for the given name is drawn as shown above.
(e)
Interpretation:
The structure for the given molecule is to be drawn.
Concept introduction:
When assigning priorities to substituents, the atom having the greater atomic number has higher priority. In case of comparison between isotopes, the one having the greater atomic mass gets higher priority. When the fourth priority substituent is pointing away (it is attached by a dash bond) and the first, second, and third priority substituents are arranged clockwise, the configuration is R. When the fourth priority substituent is pointing away (it is attached by a dash bond) and the first, second, and third priority substituents are arranged counterclockwise, the configuration is S. If the fourth priority substituent is attached by a wedge bond, then the clockwise or counterclockwise arrangement of the first, second, and third priority substituents is determined and that arrangement is reversed before assigning R or S. If the fourth priority substituent is in the plane of the page, then it is switched with the substituent that points away. Then the clockwise or counterclockwise arrangement of the first, second, and third priority substituents is determined and that arrangement is reversed before assigning R or S. When writing the IUPAC name, the R or S designation is written in parenthesis for each asymmetric carbon atom and hyphens are used to separate those designations from the rest of the IUPAC name.
Answer to Problem C.32P
The structure of
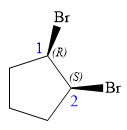
Explanation of Solution
The given name is:
In this compound, the root is cyclopentane. Two bromine atoms are attached at C1 and C2 carbon atoms of the root. The structure of the molecule without considering its stereochemistry is:
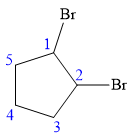
There are two chiral carbon atoms at C1 and C2 carbon atoms. The required configuration at C1 is R and that at C2 is S.
At C1 carbon atom, the four substituents are:
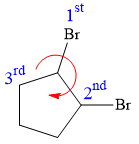
Thus, in order to have R configuration, and maintain this arrangement of substituents at the C1 carbon atom, the first-priority substituent must be attached by a wedge bond.
At C2 carbon atom, the four substituents are:
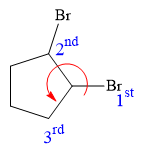
Thus, in order to have R configuration, and maintain this arrangement of substituents at the C1 carbon atom, the first-priority substituent must be attached by a dash bond.
Therefore, the correct structure for

The structure for the given name is drawn as shown above.
Want to see more full solutions like this?
Chapter C Solutions
ORGANIC CHEMISTRY E-BOOK W/SMARTWORK5
- Reaction of this bicycloalkene with bromine in carbon tetrachloride gives a trans dibromide. In both (a) and (b), the bromine atoms are trans to each other. However, only one of these products is formed. Which trans dibromide is formed? How do you account for the fact that it is formed to the exclusion of the other trans dibromide?arrow_forwardDraw the major organic product of each reaction. Indicate the stereochemistry at the stereogenic center. Omit byproducts such as salts. If applicable, expand octets to minimize formal charges.arrow_forwardExamine the following pericyclic reactions. For each reaction, tell whether it is an electrocyclic reaction, a cycloaddition reaction, or a sigmatropic rearrangement.arrow_forward
- What is the product E and how is it formed? Can this be explained using frontier molecular orbital analysis? What would the regiochemistry and stereochemistry be?arrow_forwardDisregarding stereoisomers, how many different enols can the β-diketone CH3COCH2COCH2CH3 form?arrow_forwardis dication cyclobutadiene aromatic? Based on what I know it is but im unsure about the conjugation.arrow_forward
- Consider compound I below, which is structurally related to a natural product that was isolated from an extract of a Caribbean marine sponge (See J. Chem. Soc. 1994, 116, 6015). Answer the following questions about this compound (please explain answers) How many double bonds with E stereochemistry are present? _________ How many double bonds with Z stereochemistry are present? _________arrow_forwardaddition of hbr to a double bond with an ether (-or) substituent occurs regiospecifically to give a product in which the Br OR are bonded to the same carbon. Draw the two possible carbocation intermediates in this electrophilic addition reaction,and explain using resonance why the observed product is formed.arrow_forwardThe image below shows a permitted cycloaddition reaction.?arrow_forward
- Cycloheptatrienyl radical (C7H7∙) contains a cyclic, completely conjugated system of π electrons. Is it aromatic? Is it antiaromatic? Explain.arrow_forwardClassify each of the following cycloadditions and explain why orbital symmetry conservation rules allow or forbid each of the following reactions to occur as a concerted process.arrow_forwardComplete the following reaction by including structure(s) for the product(s). The product(s) should show the correct stereochemistry.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


