8. Describe the nature of the IMF (dipole-dipole) holding ICI (iodine-chlorine) molecules together in the liquid state. Include a labeled drawing as part of your explanation.
8. Describe the nature of the IMF (dipole-dipole) holding ICI (iodine-chlorine) molecules together in the liquid state. Include a labeled drawing as part of your explanation.
ChapterU2: Smells: Molecular Structure And Properties
Section: Chapter Questions
Problem 7STP
Related questions
Question
Number 8
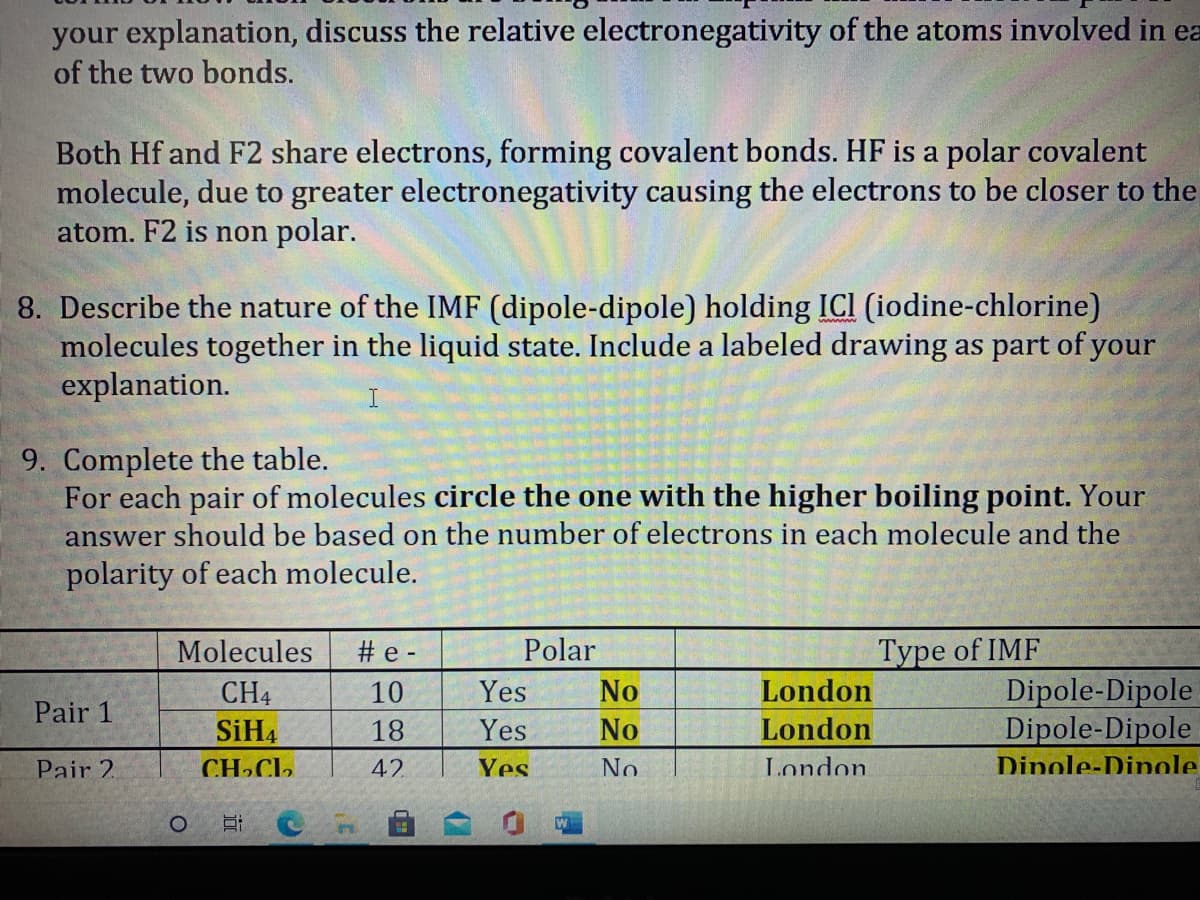
Transcribed Image Text:your explanation, discuss the relative electronegativity of the atoms involved in ea
of the two bonds.
Both Hf and F2 share electrons, forming covalent bonds. HF is a polar covalent
molecule, due to greater electronegativity causing the electrons to be closer to the
atom. F2 is non polar.
8. Describe the nature of the IMF (dipole-dipole) holding ICI (iodine-chlorine)
molecules together in the liquid state. Include a labeled drawing as part of your
explanation.
9. Complete the table.
For each pair of molecules circle the one with the higher boiling point. Your
answer should be based on the number of electrons in each molecule and the
polarity of each molecule.
Molecules
# e -
Polar
Type of IMF
London
Dipole-Dipole
Dipole-Dipole
CH4
10
Yes
No
Pair 1
SiH4
18
Yes
No
London
Pair 2.
CH»CL,
42
Yes
No
London
Dinole-Dinole
W
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning