Absorption maxima Aman ) are in nanometers, molar absorptivites (1) are in parentheses. CoH 233 (13,000) calc: 237 26 (0,500) cale: 240 291 (14,000) cale: 283 246 (15,000) oalo: 244 or 23 245 (5,500) 243 (1,400) calo: 240 240 (12,300) calo: 240 calo: 242 259 (9,00) cale: 250 300 (18,000) calo: 381 348 (40,000) oalo: 366 204 (17,000) calo: 200 270 23,000) calo: 273 or 270 225 calo: 225 276 oale: 240 262 calo: 244 200 cale: 250 311 (20,000) calo: 300 or 314
Absorption maxima Aman ) are in nanometers, molar absorptivites (1) are in parentheses. CoH 233 (13,000) calc: 237 26 (0,500) cale: 240 291 (14,000) cale: 283 246 (15,000) oalo: 244 or 23 245 (5,500) 243 (1,400) calo: 240 240 (12,300) calo: 240 calo: 242 259 (9,00) cale: 250 300 (18,000) calo: 381 348 (40,000) oalo: 366 204 (17,000) calo: 200 270 23,000) calo: 273 or 270 225 calo: 225 276 oale: 240 262 calo: 244 200 cale: 250 311 (20,000) calo: 300 or 314
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter11: Intermolecular Forces And Liquids
Section11.6: Properties Of Liquids
Problem 1.2ACP
Related questions
Question
Using Woodward–Fieser rules predict the following wavelength of the UV absorption band of the following(answer the encircled questions):
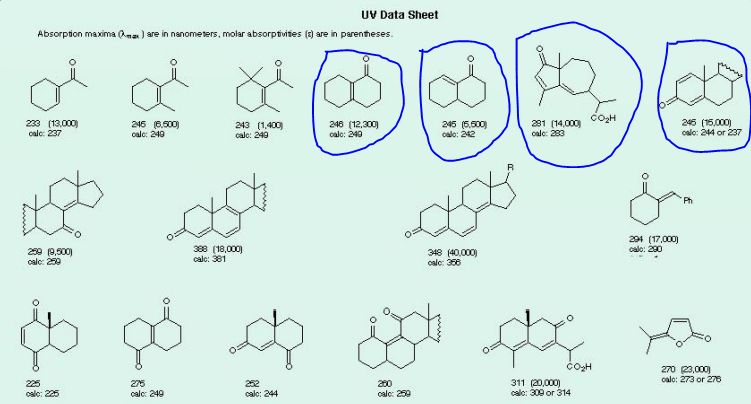
Transcribed Image Text:UV Data Sheet
Absorption maxima (Ama) are in nanometers, molar absorptivites (3) are in parentheses.
233 (13,000)
246 (6,500)
calc: 240
281 (14,000)
cale: 283
CoH
245 (15,000)
calo: 244 or 237
246 (12,300)
243 (1,400)
calc: 240
245 (5,500)
calo: 242
calc: 237
calo: 240
294 (17,000)
calo: 290
259 (9,500)
calc: 250
388 (18,000)
calc: 381
348 (40,000)
calc: 366
čoH
270 23,000)
calo: 273 or 276
311 (20,000
calc: 309 or 314
225
275
252
200
calo: 225
calc: 240
calo: 244
calc: 250
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 5 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning