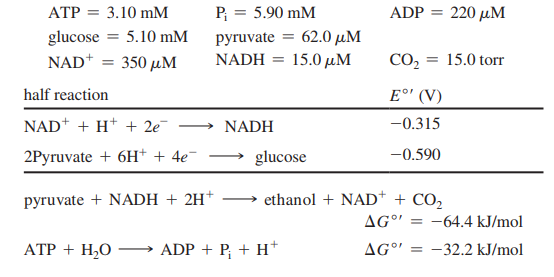
ATP = 3.10 mM P = 5.90 mM ADP = 220 µM %3D glucose = 5.10 mM NAD* = 350 µM pyruvate = 62.0 µM NADH = 15.0 µM CO, = 15.0 torr half reaction E°' (V) NAD* + H* + 2e¯ → NADH -0.315 2Pyruvate + 6H+ + 4e¯ glucose -0.590 pyruvate + NADH + 2H* ethanol + NAD* + CO2 AG°' = -64.4 kJ/mol ATP + H,O ADP + P; + H* AG°" -32.2 kJ/mol %3D
For part (b) of this problem, use the following standard reduction potentials, free energies, and nonequilibrium concentrations of reactants and products: Consider the last two steps in the alcoholic fermentation of glucose by brewer’s yeast: pyruvate + NADH + 2H+ → ethanol + NAD+ + CO2
(a) Do you predict that ∆S° for this reaction is > 0 or < 0?
(b) Calculate the nonequilibrium concentration of ethanol in yeast cells, if ∆G = -38.3 kJ/mol for this reaction at pH = 7.4 and 37 °C when the reactants and products are at the concentrations given above.
(c) How would a drop in pH affect ∆G for the reaction described in part (b)?
(d) How would an increase in intracellular CO2 levels affect ∆G for the reaction in part (b)?
(e) How would an increase in intracellular CO2 levels affect ∆G°′ for the reaction in part (b)?
Trending now
This is a popular solution!
Step by step
Solved in 3 steps









