B. Titration Data A 100 mL Kool-Aid solution was made: 0.3210 g of Kool-Aid powder was quantitatively transferred to 100 mL volumetric flask. The flask was filled to the meniscus to the calibration mark. 1. 2. 10.00 mL aliquots of this solution were used for each titration. The concentration of the sodium hydroxide titrant was 0.02468 M. Three drops of phenolphthalein indicator were used for each titration. 3. Four titrations were performed. The initial and final volumes of the sodium hydroxide solution for each trial are given in Table 1. Table 1. Titration Data Trial 1 Trial 2 Trial 3 Trial 4 Initial NaOH volume 0.24 (mL) Final NaOH volume (mL) NAOH volume used (mL) 17.26 0.13 17.32 17.46 34.50 17.32 34.53
B. Titration Data A 100 mL Kool-Aid solution was made: 0.3210 g of Kool-Aid powder was quantitatively transferred to 100 mL volumetric flask. The flask was filled to the meniscus to the calibration mark. 1. 2. 10.00 mL aliquots of this solution were used for each titration. The concentration of the sodium hydroxide titrant was 0.02468 M. Three drops of phenolphthalein indicator were used for each titration. 3. Four titrations were performed. The initial and final volumes of the sodium hydroxide solution for each trial are given in Table 1. Table 1. Titration Data Trial 1 Trial 2 Trial 3 Trial 4 Initial NaOH volume 0.24 (mL) Final NaOH volume (mL) NAOH volume used (mL) 17.26 0.13 17.32 17.46 34.50 17.32 34.53
Chapter16: Applications Of Neutralization Titrations
Section: Chapter Questions
Problem 16.40QAP
Related questions
Question
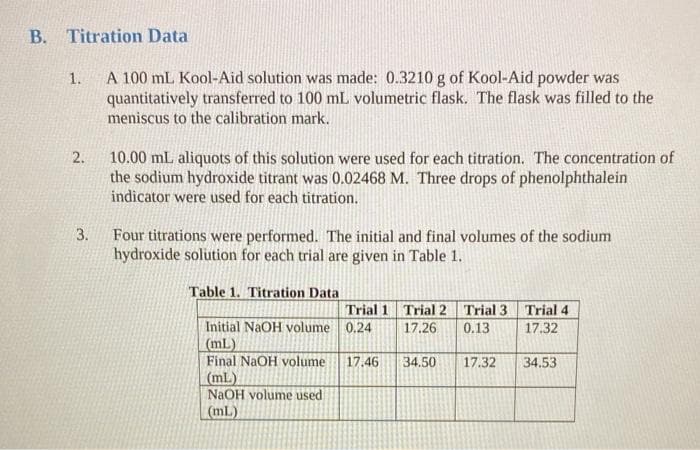
Transcribed Image Text:B. Titration Data
A 100 mL Kool-Aid solution was made: 0.3210 g of Kool-Aid powder was
quantitatively transferred to 100 mL volumetric flask. The flask was filled to the
meniscus to the calibration mark.
1.
2.
10.00 mL aliquots of this solution were used for each titration. The concentration of
the sodium hydroxide titrant was 0.02468 M. Three drops of phenolphthalein
indicator were used for each titration.
3.
Four titrations were performed. The initial and final volumes of the sodium
hydroxide solution for each trial are given in Table 1.
Table 1. Titration Data
Trial 1 Trial 2 Trial 3 Trial4
Initial NaOH volume 0.24
(mL)
Final NaOH volume
(mL)
NAOH volume used
17.26
0.13
17.32
17.46
34.50
17.32
34.53
(mL)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



