Both glucose (corn sugar) and fructose (fruit sugar) taste sweet, but fructose tastes sweeter. Each year in the United States, tons of corn syrup destined to sweeten food are treated to convert glucose as fully as possible to the sweeter fructose. The reaction is an equilibrium: glucose 2 fructose (a) A 0.2564 M solution of pure glucose is treated at 25°C with an enzyme (catalyst) that causes the pre- ceding equilibrium to be reached quickly. The final concentration of fructose is 0.1175 M. In another experiment at the same temperature, a 0.2666 M solu- tion of pure fructose is treated with the same enzyme and the final concentration of glucose is 0.1415 M. Compute an average equilibrium constant for the preceding reaction. (b) At equilibrium under these conditions, what percent- age of glucose is converted to fructose?
Both glucose (corn sugar) and fructose (fruit sugar) taste sweet, but fructose tastes sweeter. Each year in the United States, tons of corn syrup destined to sweeten food are treated to convert glucose as fully as possible to the sweeter fructose. The reaction is an equilibrium: glucose 2 fructose (a) A 0.2564 M solution of pure glucose is treated at 25°C with an enzyme (catalyst) that causes the pre- ceding equilibrium to be reached quickly. The final concentration of fructose is 0.1175 M. In another experiment at the same temperature, a 0.2666 M solu- tion of pure fructose is treated with the same enzyme and the final concentration of glucose is 0.1415 M. Compute an average equilibrium constant for the preceding reaction. (b) At equilibrium under these conditions, what percent- age of glucose is converted to fructose?
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter14: Chemical Equilibrium
Section: Chapter Questions
Problem 88AP
Related questions
Question
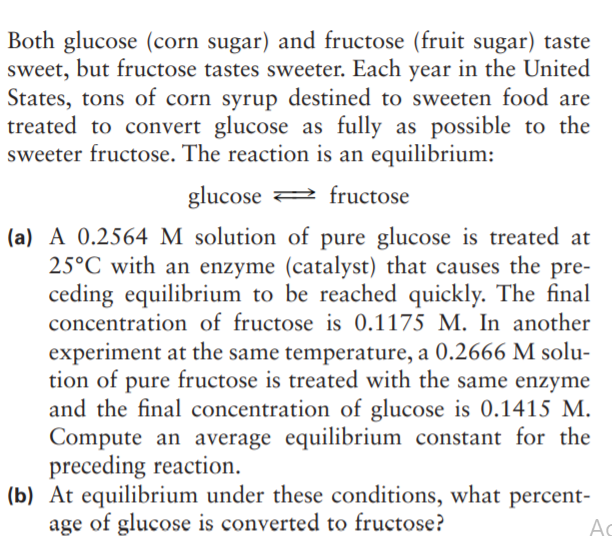
Transcribed Image Text:Both glucose (corn sugar) and fructose (fruit sugar) taste
sweet, but fructose tastes sweeter. Each year in the United
States, tons of corn syrup destined to sweeten food are
treated to convert glucose as fully as possible to the
sweeter fructose. The reaction is an equilibrium:
glucose 2 fructose
(a) A 0.2564 M solution of pure glucose is treated at
25°C with an enzyme (catalyst) that causes the pre-
ceding equilibrium to be reached quickly. The final
concentration of fructose is 0.1175 M. In another
experiment at the same temperature, a 0.2666 M solu-
tion of pure fructose is treated with the same enzyme
and the final concentration of glucose is 0.1415 M.
Compute an average equilibrium constant for the
preceding reaction.
(b) At equilibrium under these conditions, what percent-
age of glucose is converted to fructose?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 6 steps

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning