(c) Mathematically determine the ro and Eo values using the solutions to Problem 2.18 and compare these with the graphical results from part (b). ro= Eo = 0.24 i -5.32 nm eV
(c) Mathematically determine the ro and Eo values using the solutions to Problem 2.18 and compare these with the graphical results from part (b). ro= Eo = 0.24 i -5.32 nm eV
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter5: Quantum Mechanics And Atomic Structure
Section: Chapter Questions
Problem 55AP: The outermost electron in an alkali-metal atom is sometimes described as resembling an electron in...
Related questions
Question

Transcribed Image Text:For a K*-CI-ion pair, attractive and repulsive energies EA and ER, respectively, depend on the distance between the ions r, according to
-1.436
EA
ER
=
r
5.86 x 10-6
For these expressions, energies are expressed in electron volts per K+-CI pair, and r is the distance in nanometers. The net energy En
is just the sum of the preceding two expressions.
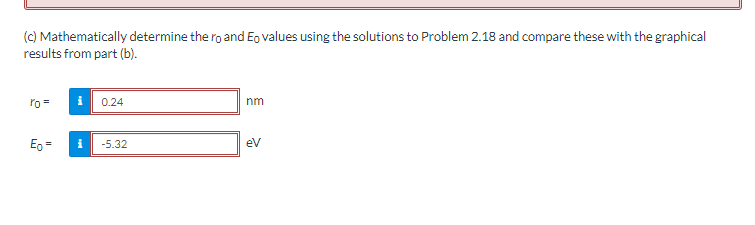
Transcribed Image Text:(c) Mathematically determine the ro and Eo values using the solutions to Problem 2.18 and compare these with the graphical
results from part (b).
ro=
Eo =
0.24
-5.32
nm
eV
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning