Write the mass-action expression, Qc, for the following chemical reaction equations: 15 1) CaCO3(s) <-> CaO(s) + CO2(g) 2) CH4(g) + 202(g) <-> CO2(g) + 2H20(1) 3) AgCI(s) Ag* (aq) + Cl" く-> (aq) 4) Zn(s) + Cu2*(aq) Zn2+ <-> (aq) + Cu(s)
Write the mass-action expression, Qc, for the following chemical reaction equations: 15 1) CaCO3(s) <-> CaO(s) + CO2(g) 2) CH4(g) + 202(g) <-> CO2(g) + 2H20(1) 3) AgCI(s) Ag* (aq) + Cl" く-> (aq) 4) Zn(s) + Cu2*(aq) Zn2+ <-> (aq) + Cu(s)
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter11: Atomic Mass Spectrometry
Section: Chapter Questions
Problem 11.10QAP
Related questions
Question
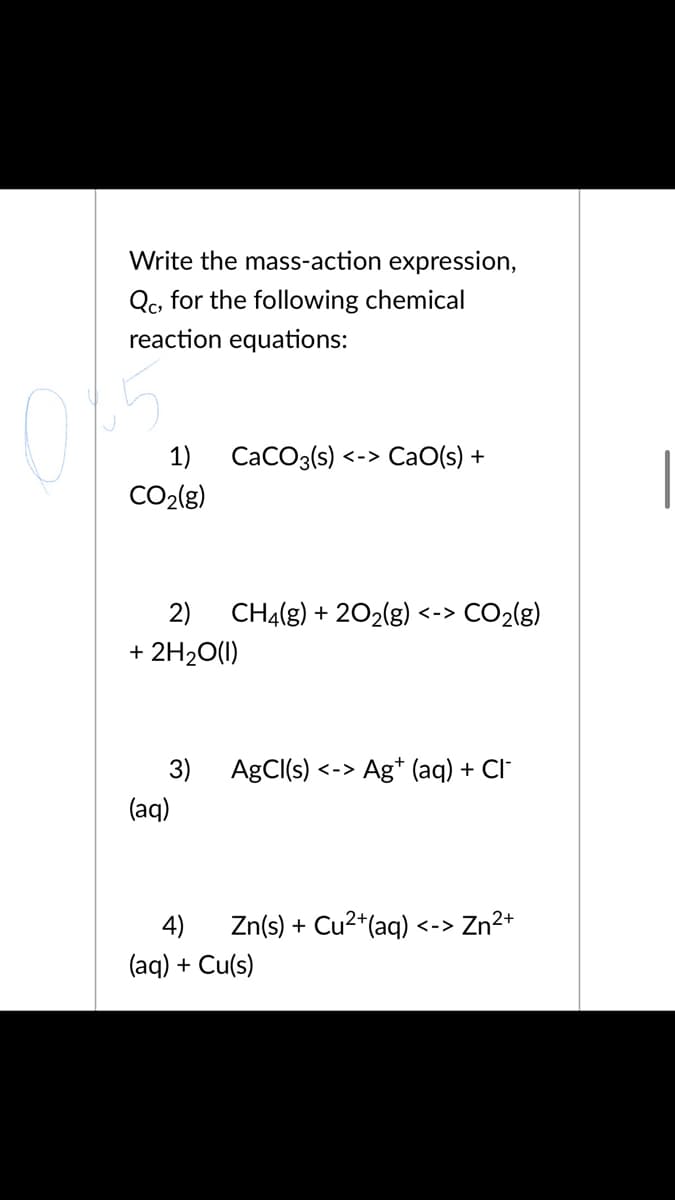
Transcribed Image Text:Write the mass-action expression,
Qc, for the following chemical
reaction equations:
15
1)
CaCO3(s) <-> CaO(s) +
CO2(g)
2)
CH4(g) + 202(g) <-> CO2(g)
+ 2H20(1)
3) AgCI(s)
Ag* (aq) + Cl"
く->
(aq)
4)
Zn(s) + Cu2*(aq)
Zn2+
<->
(aq) + Cu(s)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning
