Home 101 Chem 101 My Questions bartleby X (274) Banda Carnaval - Sueñ X X X app.101edu.co Unofficial Transcript... Oregon Scholarship.... Welcome to the OS... myClackamas Login Document Require... Apps WLogon Home FAFSA on t... The National Societ... > Submit Question 5 of 20 If 450 g of magnesium hydroxide is dissolved in water to make 6.5 L of solution, what is the concentration in mM? mM 2 1 4 5 6 с 7 8 +- 0 x 100 5:05 PM Type here to search о ENG 11/21/2019 LO
Home 101 Chem 101 My Questions bartleby X (274) Banda Carnaval - Sueñ X X X app.101edu.co Unofficial Transcript... Oregon Scholarship.... Welcome to the OS... myClackamas Login Document Require... Apps WLogon Home FAFSA on t... The National Societ... > Submit Question 5 of 20 If 450 g of magnesium hydroxide is dissolved in water to make 6.5 L of solution, what is the concentration in mM? mM 2 1 4 5 6 с 7 8 +- 0 x 100 5:05 PM Type here to search о ENG 11/21/2019 LO
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter34: Particle Size Determination
Section: Chapter Questions
Problem 34.12QAP
Related questions
Question
Transcribed Image Text:Home
101 Chem 101
My Questions bartleby
X
(274) Banda Carnaval - Sueñ
X
X
X
app.101edu.co
Unofficial Transcript...
Oregon Scholarship....
Welcome to the OS...
myClackamas Login
Document Require...
Apps WLogon
Home FAFSA on t...
The National Societ...
>
Submit
Question 5 of 20
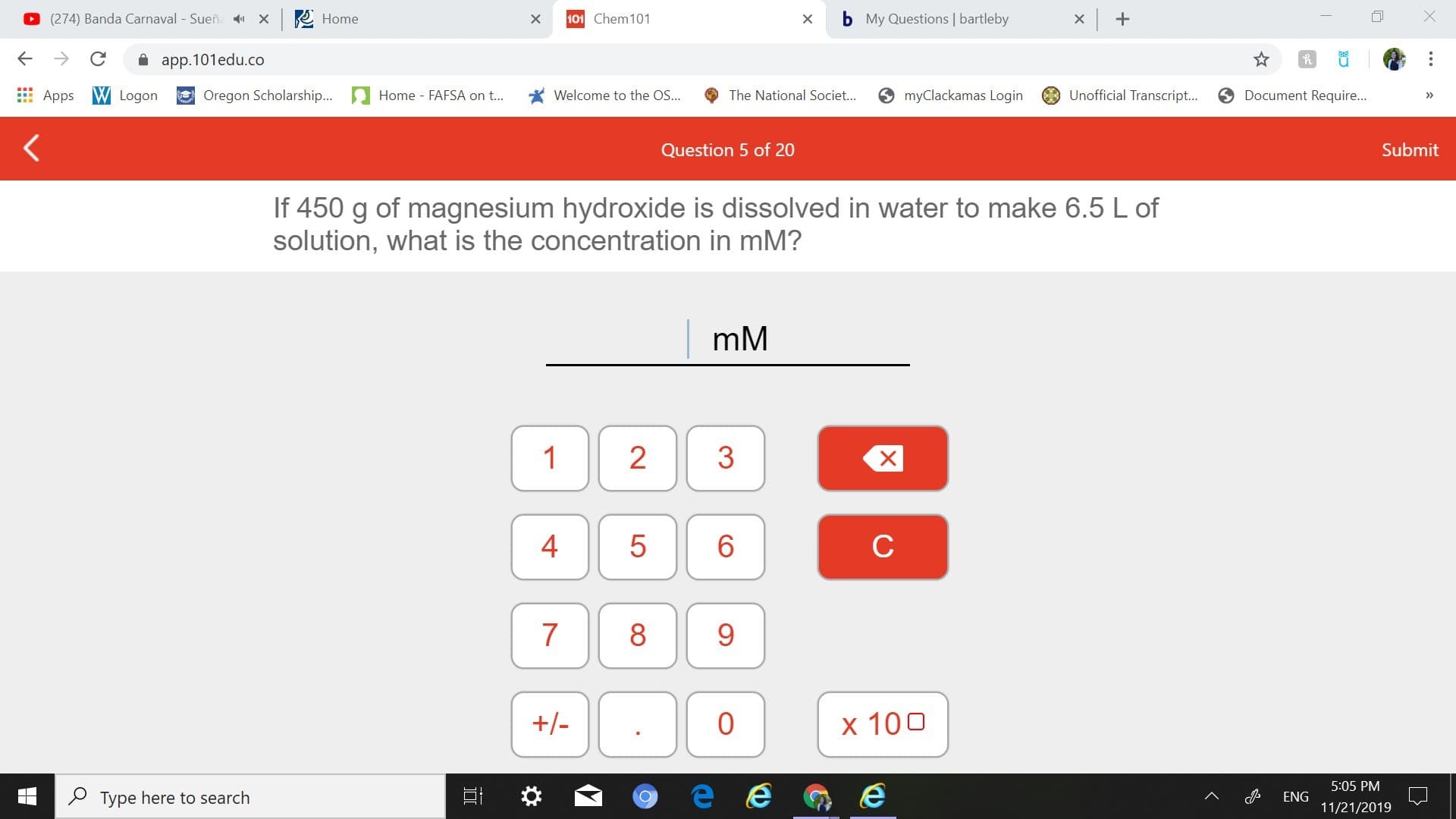
If 450 g of magnesium hydroxide is dissolved in water to make 6.5 L of
solution, what is the concentration in mM?
mM
2
1
4
5
6
с
7
8
+-
0
x 100
5:05 PM
Type here to search
о
ENG
11/21/2019
LO
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning