In a titration of unknown triprotic acid, H3A, 63.5 mL of 0.556 M calcium hydroxide are needed to neutralize 25.0 mL of the acid. Which is the balanced chemical reaction for this process? Note that A represents any anion with -3 charge. О 2НЗА(aq) + 3Саa(ОН)2(aq) — Саз А2(ag) + 6H20(0 НзА(ag) + 3CаОН(aq) — зСаА(aq) + зн200 НзА(аq) + CаOH(aq) — СаА (аq) + H20Ф H3A(aq) + Ca(OH)2(aq) → CaA2(aq) + H20(1) HзА(ag) + Ca(ОН)2(ag) — Саз А2(aq) + H200
In a titration of unknown triprotic acid, H3A, 63.5 mL of 0.556 M calcium hydroxide are needed to neutralize 25.0 mL of the acid. Which is the balanced chemical reaction for this process? Note that A represents any anion with -3 charge. О 2НЗА(aq) + 3Саa(ОН)2(aq) — Саз А2(ag) + 6H20(0 НзА(ag) + 3CаОН(aq) — зСаА(aq) + зн200 НзА(аq) + CаOH(aq) — СаА (аq) + H20Ф H3A(aq) + Ca(OH)2(aq) → CaA2(aq) + H20(1) HзА(ag) + Ca(ОН)2(ag) — Саз А2(aq) + H200
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter4: Stoichiometry Of Chemical Reactions
Section: Chapter Questions
Problem 79E: Titration of a 20.0-mL sample of acid rain required 1.7 mL of 0.08 11 M NaOH to reach the end point....
Related questions
Question
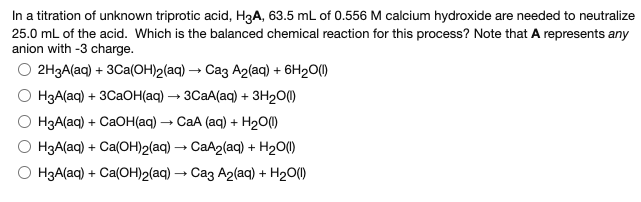
Transcribed Image Text:In a titration of unknown triprotic acid, H3A, 63.5 mL of 0.556 M calcium hydroxide are needed to neutralize
25.0 mL of the acid. Which is the balanced chemical reaction for this process? Note that A represents any
anion with -3 charge.
О 2НЗА(aq) + 3Саa(ОН)2(aq) — Саз А2(ag) + 6H20(0
НзА(ag) + 3CаОН(aq) — зСаА(aq) + зн200
НзА(аq) + CаOH(aq) — СаА (аq) + H20Ф
H3A(aq) + Ca(OH)2(aq) → CaA2(aq) + H20(1)
HзА(ag) + Ca(ОН)2(ag) — Саз А2(aq) + H200
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning