it into a glass tube filled with 40 ml of water (final volume). What is the amount of dye present in the You have a stock dye solution with a concentration of 0.25 mg/ml. You take 20 µl of this dye and place glass tube?
it into a glass tube filled with 40 ml of water (final volume). What is the amount of dye present in the You have a stock dye solution with a concentration of 0.25 mg/ml. You take 20 µl of this dye and place glass tube?
Anatomy & Physiology
1st Edition
ISBN:9781938168130
Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark Womble
Publisher:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark Womble
Chapter26: Fluid, Electrolyte, And Acid-base Balance
Section: Chapter Questions
Problem 6RQ: A cation has a(n) ________ charge. neutral positive alternating negative
Related questions
Question
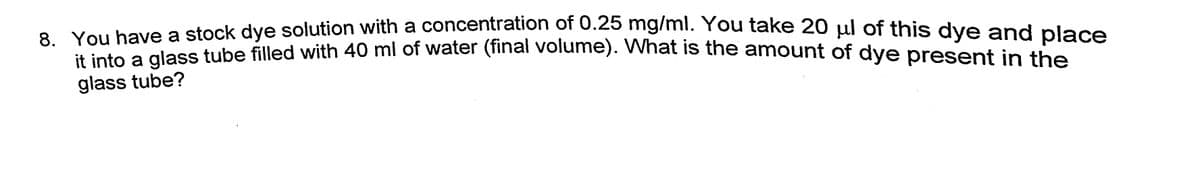
Transcribed Image Text:8 You have a stock dye solution with a concentration of 0.25 mg/mi. You take 20 ul of this dve and place
You nave ses tube filled with 40 ml of water (final volume). What is the amount of dye present in the
glass tube?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Anatomy & Physiology
Biology
ISBN:
9781938168130
Author:
Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark Womble
Publisher:
OpenStax College

Anatomy & Physiology
Biology
ISBN:
9781938168130
Author:
Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark Womble
Publisher:
OpenStax College