Moles of Element X I Moles of Element Y Moles of Element Z 2.25 2.00 1.75 1.50 1.25 1.00 0.75 0.50 0.25 0.00 50 100 150 200 Time (seconds) Based on the chart above, which reactant is limiting? Moles of reactant or product
Moles of Element X I Moles of Element Y Moles of Element Z 2.25 2.00 1.75 1.50 1.25 1.00 0.75 0.50 0.25 0.00 50 100 150 200 Time (seconds) Based on the chart above, which reactant is limiting? Moles of reactant or product
Chemistry for Today: General, Organic, and Biochemistry
9th Edition
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Chapter5: Chemical Reactions
Section: Chapter Questions
Problem 5.43E: Calculate the number of moles of CO2 generated by the reaction of Exercise 5.42 when 500.g of CaO is...
Related questions
Question
100%
help
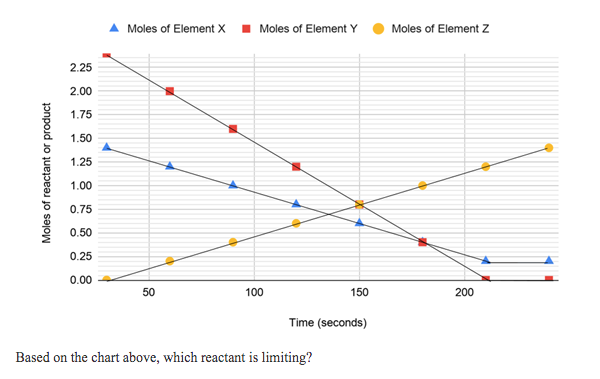
Transcribed Image Text:Moles of Element X
I Moles of Element Y
Moles of Element Z
2.25
2.00
1.75
1.50
1.25
1.00
0.75
0.50
0.25
0.00
50
100
150
200
Time (seconds)
Based on the chart above, which reactant is limiting?
Moles of reactant or product

Transcribed Image Text:Substance X, because it is the reactant that was completely used up
Substance Y, because it is the reactant that was completely used up
Substance X, because it is the only reactant that is leftover
Substance Y, because it is the only reactant that is leftover
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT


Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning