Note: 10 drops of red dye is equal to 0.50 mL, and the initial concentration of the FD&C red dye in the pipet is 0.0201 M. Review the following equation M1V1 = M2V2 , where M1 is the intial concentration, V1 is the intial volume, M2 is the final concentration and V2 is the final concentration. Use the M1V1 = M2V2 equation to calculate the final concentration of red dye in each of the test tubes. Record the final concentrations for each test tube
Note: 10 drops of red dye is equal to 0.50 mL, and the initial concentration of the FD&C red dye in the pipet is 0.0201 M. Review the following equation M1V1 = M2V2 , where M1 is the intial concentration, V1 is the intial volume, M2 is the final concentration and V2 is the final concentration. Use the M1V1 = M2V2 equation to calculate the final concentration of red dye in each of the test tubes. Record the final concentrations for each test tube
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter4: Types Of Chemical Reactions And Solution Stoichiometry
Section: Chapter Questions
Problem 70E: You are given a 1.50-g mixture of sodium nitrate and sodium chloride. You dissolve this mixture into...
Related questions
Question
Note: 10 drops of red dye is equal to 0.50 mL, and the initial concentration of the FD&C red dye in the pipet is 0.0201 M.
Review the following equation M1V1 = M2V2 , where M1 is the intial concentration, V1 is the intial volume, M2 is the final concentration and V2 is the final concentration.
Use the M1V1 = M2V2 equation to calculate the final concentration of red dye in each of the test tubes. Record the final concentrations for each test tube.
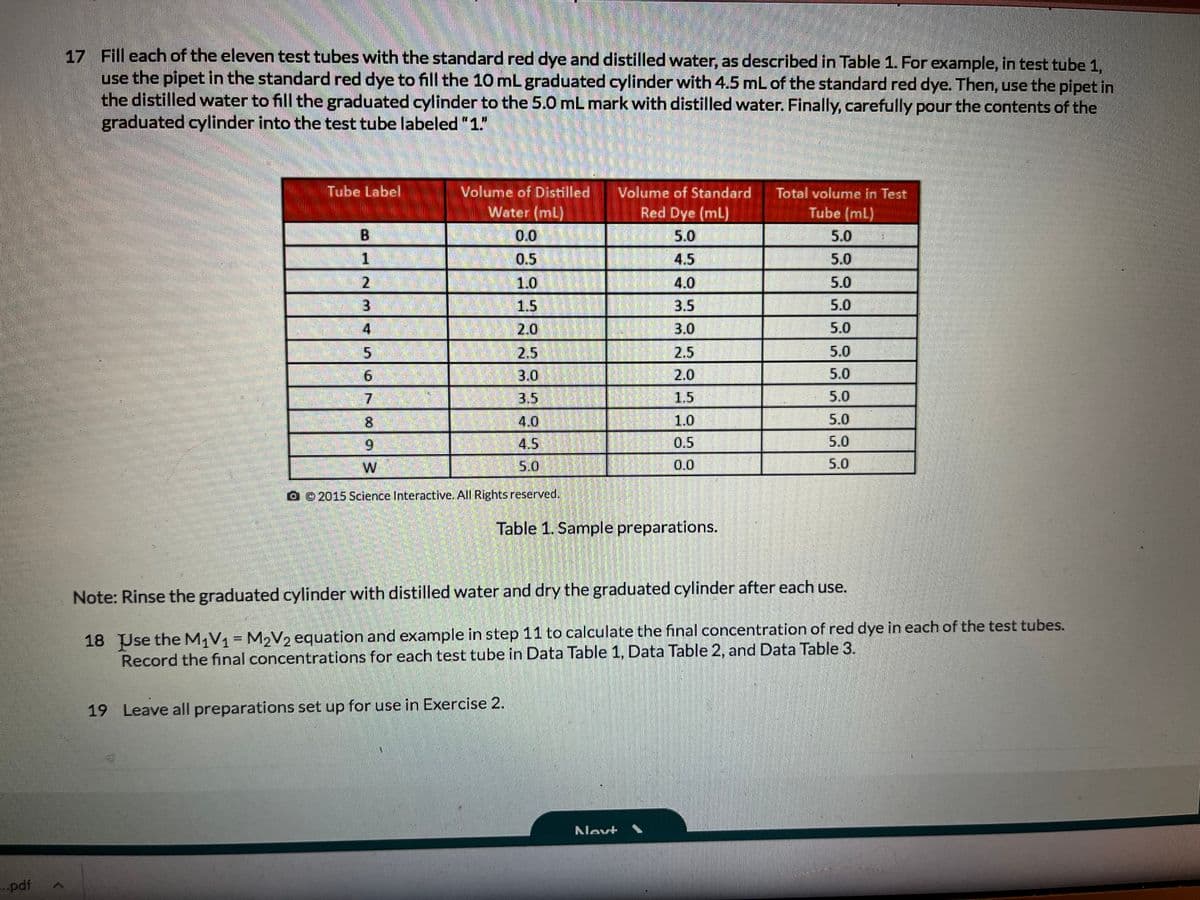
Transcribed Image Text:17 Fill each of the eleven test tubes with the standard red dye and distilled water, as described in Table 1. For example, in test tube 1,
use the pipet in the standard red dye to fill the 10 mL graduated cylinder with 4.5 mL of the standard red dye. Then, use the pipet in
the distilled water to fill the graduated cylinder to the 5.0 mL mark with distilled water. Finally, carefully pour the contents of the
graduated cylinder into the test tube labeled 1"
Tube Label
Volume of Distilled
Volume of Standard
Total volume in Test
Tube (ml)
Water (mL)
Red Dye (mL)
0.0
5.0
5.0
1.
0.5
4.5
5.0
2.
1.0
4.0
5.0
3
1.5
3.5
5.0
2.0
3.0
5.0
2.5
2.5
5.0
3.0
2.0
5.0
7.
R3.5
1.5
5.0
5.0
4.0
4.5)
8.
1.0
6.
0.5
5.0
W
5.0
0.0
5.0
0 0 2015 Science Interactive. All Rights reserved.
Table 1. Sample preparations.
Note: Rinse the graduated cylinder with distilled water and dry the graduated cylinder after each use.
18 Use the M1V1= M2V2 equation and example in step 11 to calculate the final concentration of red dye in each of the test tubes.
Record the final concentrations for each test tube in Data Table 1, Data Table 2, and Data Table 3.
%3D
19 Leave all preparations set up for use in Exercise 2.
Nevt
--.pdf
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning