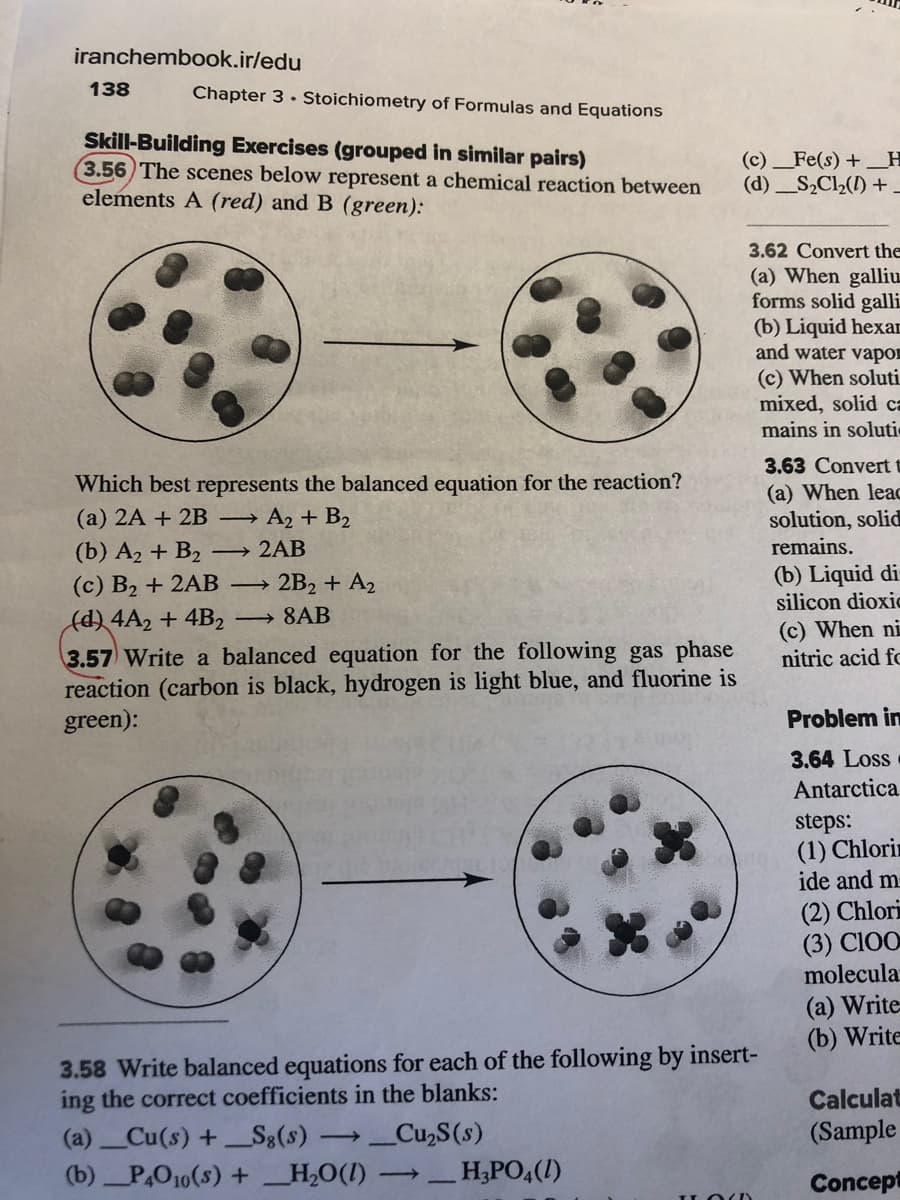
Skill-Building Exercises (grouped in similar pairs) 3.56 The scenes below represent a chemical reaction between elements A (red) and B (green): Which best represents the balanced equation for the reaction? (a) 2A + 2B → A2 + B2 (b) A2 + B2 (c) B2 + 2AB → 2B2 + A2 - 2AB > (d) 4A2 + 4B2 8AB -
Skill-Building Exercises (grouped in similar pairs) 3.56 The scenes below represent a chemical reaction between elements A (red) and B (green): Which best represents the balanced equation for the reaction? (a) 2A + 2B → A2 + B2 (b) A2 + B2 (c) B2 + 2AB → 2B2 + A2 - 2AB > (d) 4A2 + 4B2 8AB -
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter3: Calculations With Chemical Formulas And Equaitons
Section: Chapter Questions
Problem 3.141QP: A power plant is driven by the combustion of a complex fossil fuel having the formula C11H7S. Assume...
Related questions
Question
Number 56 please
Transcribed Image Text:iranchembook.ir/edu
138
Chapter 3 • Stoichiometry of Formulas and Equations
Skill-Building Exercises (grouped in similar pairs)
3.56 The scenes below represent a chemical reaction between
elements A (red) and B (green):
(c) _Fe(s) + _H
(d) _S,Cl(1) +-
3.62 Convert the
(a) When galliu.
forms solid galli
(b) Liquid hexar
and water vapon
(c) When soluti
mixed, solid ca
mains in soluti
3.63 Convert t
Which best represents the balanced equation for the reaction?
(a) 2A + 2B → A2 + B2
(a) When lea
solution, solid
remains.
(b) A2 + B2
→ 2AB
2B2 + A2
(b) Liquid di
silicon dioxic
(с) В2 + 2AВ —
(d) 4A2 + 4B2
3.57 Write a balanced equation for the following gas phase
reaction (carbon is black, hydrogen is light blue, and fluorine is
green):
→ 8AB
(c) When ni
nitric acid fc
Problem in
3.64 Loss
Antarctica
steps:
(1) Chlorin
ide and m
(2) Chlori
(3) CIOO
molecula
(a) Write
(b) Write
3.58 Write balanced equations for each of the following by insert-
ing the correct coefficients in the blanks:
Calculat
(a)Cu(s) +S8(s)
_Cu,S(s)
(Sample
(b)P,O10(s) + _H,O(I)
_H;PO,(1)
>
Concept
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning