The figures below are of temperature monitos with blue fluid in which a tube (that was also been filled with the blue fluid has been inverted and placed in the beaker (open on the end that's submerged in the blue fluid). As the temperature is raised on the beaker/tube system that level of blue fluid in the tube will transition A completely full
The figures below are of temperature monitos with blue fluid in which a tube (that was also been filled with the blue fluid has been inverted and placed in the beaker (open on the end that's submerged in the blue fluid). As the temperature is raised on the beaker/tube system that level of blue fluid in the tube will transition A completely full
Chemistry for Today: General, Organic, and Biochemistry
9th Edition
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Chapter6: The States Of Matter
Section: Chapter Questions
Problem 6.70E
Related questions
Question
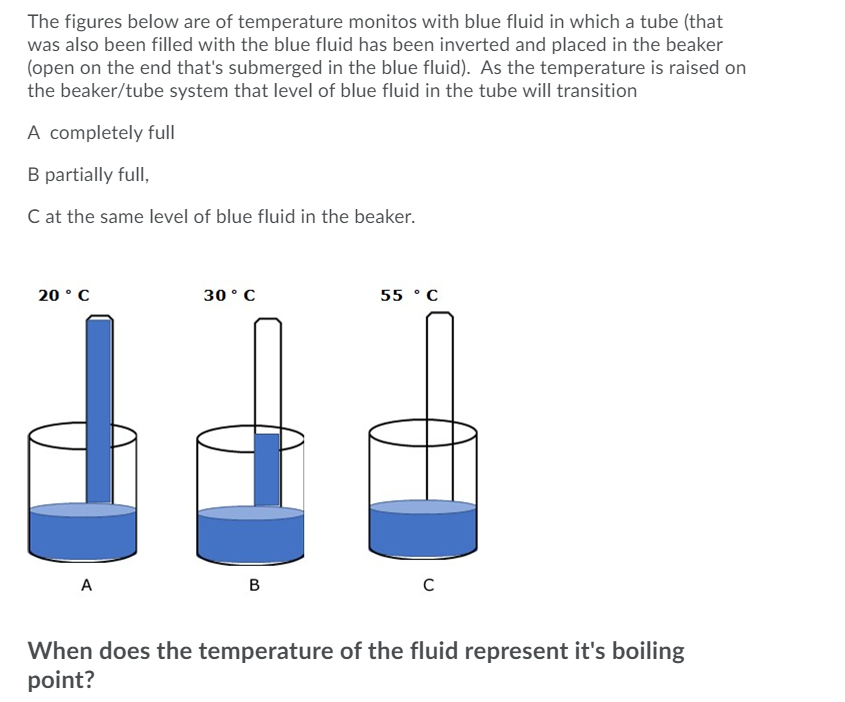
Transcribed Image Text:The figures below are of temperature monitos with blue fluid in which a tube (that
was also been filled with the blue fluid has been inverted and placed in the beaker
(open on the end that's submerged in the blue fluid). As the temperature is raised on
the beaker/tube system that level of blue fluid in the tube will transition
A completely full
B partially full,
C at the same level of blue fluid in the beaker.
20 °C
30° C
55 °C
A
B
C
When does the temperature of the fluid represent it's boiling
point?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,