The kinetic data for the activity of 0.01mM enzyme is shown below. The concentration of {S} is millimolar and reaction rate is millimolar per second. Take this information down. Produce a Lineweaver-Burke graph using the data below, find the Km, find the Vm, and calculate the kcat for this enzyme. Turn in a single page with the graph (title, axes labels and units, etc) and the values of Km, Vm, and kcat (see the example image below)
The kinetic data for the activity of 0.01mM enzyme is shown below. The concentration of {S} is millimolar and reaction rate is millimolar per second. Take this information down. Produce a Lineweaver-Burke graph using the data below, find the Km, find the Vm, and calculate the kcat for this enzyme. Turn in a single page with the graph (title, axes labels and units, etc) and the values of Km, Vm, and kcat (see the example image below)
Biochemistry
9th Edition
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Chapter1: Biochemistry: An Evolving Science
Section: Chapter Questions
Problem 1P
Related questions
Question
Can someone help w/this please?

Transcribed Image Text:An enzyme reacts with substrate S to produce
P.
E +SS ES → E + P
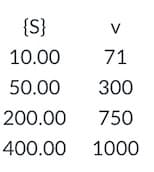
The kinetic data for the activity of 0.01mM
enzyme is shown below. The concentration of
{S} is millimolar and reaction rate is millimolar
per second. Take this information down.
Produce a Lineweaver-Burke graph using the
data below, find the Km, find the Vm, and
calculate the kcat for this enzyme. Turn in a
single page with the graph (title, axes labels
and units, etc) and the values of Km, Vm, and
kcat (see the example image below)
Name: J Doe
Lineweaver-Burke Graph for Enzyme X
1/v
(s/mM)
1/s
(/mM)
K - ?
keat - ?
Transcribed Image Text:{S}
V
10.00
71
50.00
300
200.00
750
400.00
1000
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

Biochemistry
Biochemistry
ISBN:
9781319114671
Author:
Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:
W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:
9781464126116
Author:
David L. Nelson, Michael M. Cox
Publisher:
W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul…
Biochemistry
ISBN:
9781118918401
Author:
Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:
WILEY

Biochemistry
Biochemistry
ISBN:
9781319114671
Author:
Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:
W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:
9781464126116
Author:
David L. Nelson, Michael M. Cox
Publisher:
W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul…
Biochemistry
ISBN:
9781118918401
Author:
Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:
WILEY

Biochemistry
Biochemistry
ISBN:
9781305961135
Author:
Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:
Cengage Learning

Biochemistry
Biochemistry
ISBN:
9781305577206
Author:
Reginald H. Garrett, Charles M. Grisham
Publisher:
Cengage Learning

Fundamentals of General, Organic, and Biological …
Biochemistry
ISBN:
9780134015187
Author:
John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:
PEARSON