The slightly soluble salt fluorapatite, Cas(PO4)3F, has a Ksp = 1 x 1031. It dissociates in water according to the following equation: Cas(PO4)3F(s) 5Ca2+ +3 PO43- + F Write the Ksp equation for this process: Ksp = 1. 2. Calculate the equilibrium concentrations of Ca2+, PO43- , and F. a. [Ca2] = b. [PO43-]= c. [F] = How many grams of fluorapatite will dissolve in 1L of water? 3. 4. The solubility of the related salt hydroxyapatite, Cas(PO4)30H (Ksp 1 x 10-29), is affected by the pH of the water to which it is added. Calculate the number of grams of hydroxyapatite that will dissolve in 0.250L of a. neutral water. Calculate the number of grams of hydroxyapatite that will dissolve in 0.250L of b. water having a pH 5.5 Thus, the equilibrium concentration of ions is, =5x = 5x 1.01x104-5.05x104 Ca4 POj3x3x1.01x 10 -3.03x104 [Fx 1.01x10-4
The slightly soluble salt fluorapatite, Cas(PO4)3F, has a Ksp = 1 x 1031. It dissociates in water according to the following equation: Cas(PO4)3F(s) 5Ca2+ +3 PO43- + F Write the Ksp equation for this process: Ksp = 1. 2. Calculate the equilibrium concentrations of Ca2+, PO43- , and F. a. [Ca2] = b. [PO43-]= c. [F] = How many grams of fluorapatite will dissolve in 1L of water? 3. 4. The solubility of the related salt hydroxyapatite, Cas(PO4)30H (Ksp 1 x 10-29), is affected by the pH of the water to which it is added. Calculate the number of grams of hydroxyapatite that will dissolve in 0.250L of a. neutral water. Calculate the number of grams of hydroxyapatite that will dissolve in 0.250L of b. water having a pH 5.5 Thus, the equilibrium concentration of ions is, =5x = 5x 1.01x104-5.05x104 Ca4 POj3x3x1.01x 10 -3.03x104 [Fx 1.01x10-4
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter15: Equilibria Of Other Reaction Classes
Section: Chapter Questions
Problem 109E: The following question is taken from a Chemistry Advanced Placement Examination and is used with the...
Related questions
Question
How to answer question 4?
![The slightly soluble salt fluorapatite, Cas(PO4)3F, has a Ksp = 1 x 1031. It dissociates in water
according to the following equation:
Cas(PO4)3F(s) 5Ca2+ +3 PO43-
+ F
Write the Ksp equation for this process: Ksp =
1.
2. Calculate the equilibrium concentrations of Ca2+, PO43- , and F.
a. [Ca2] =
b. [PO43-]=
c. [F] =
How many grams of fluorapatite will dissolve in 1L of water?
3.
4. The solubility of the related salt hydroxyapatite, Cas(PO4)30H (Ksp 1 x 10-29), is affected
by the pH of the water to which it is added.
Calculate the number of grams of hydroxyapatite that will dissolve in 0.250L of
a.
neutral water.
Calculate the number of grams of hydroxyapatite that will dissolve in 0.250L of
b.
water having a pH
5.5](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F222ac239-1f16-4a01-8023-c69ec7876b73%2Fd454bf6a-cd94-4fcf-8d3d-ef5fea72f11e%2Fsyicv9j.jpeg&w=3840&q=75)
Transcribed Image Text:The slightly soluble salt fluorapatite, Cas(PO4)3F, has a Ksp = 1 x 1031. It dissociates in water
according to the following equation:
Cas(PO4)3F(s) 5Ca2+ +3 PO43-
+ F
Write the Ksp equation for this process: Ksp =
1.
2. Calculate the equilibrium concentrations of Ca2+, PO43- , and F.
a. [Ca2] =
b. [PO43-]=
c. [F] =
How many grams of fluorapatite will dissolve in 1L of water?
3.
4. The solubility of the related salt hydroxyapatite, Cas(PO4)30H (Ksp 1 x 10-29), is affected
by the pH of the water to which it is added.
Calculate the number of grams of hydroxyapatite that will dissolve in 0.250L of
a.
neutral water.
Calculate the number of grams of hydroxyapatite that will dissolve in 0.250L of
b.
water having a pH
5.5
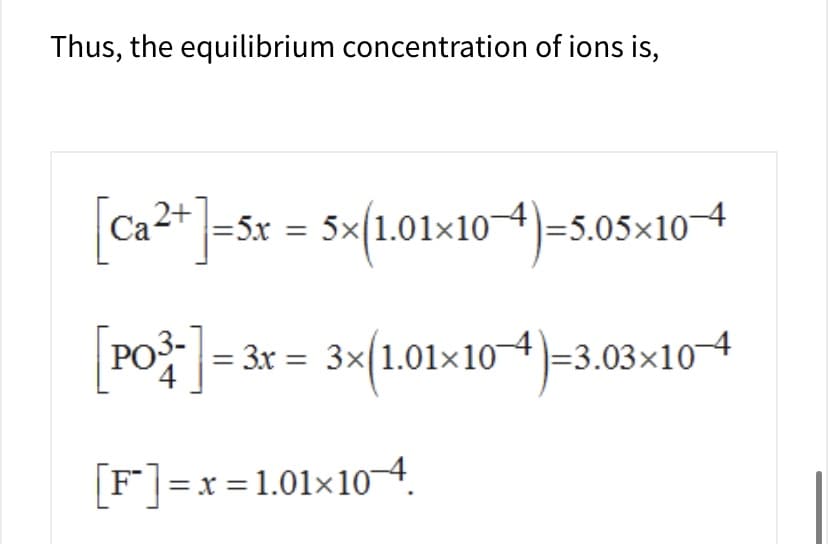
Transcribed Image Text:Thus, the equilibrium concentration of ions is,
=5x = 5x 1.01x104-5.05x104
Ca4
POj3x3x1.01x 10
-3.03x104
[Fx 1.01x10-4
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning