The van der Waals equation of state was designed (by Dutch physicist Johannes van der Waals) to predict the relationship between pressure p, volume V and temperature T for gases better than the Ideal Gas Law does: p+a (V-nb)=nRT The van der Waals equation of state. R stands for the gas constant and n for moles of gas. The parameters a and b must be determined for each gas from experimental data. Use the van der Waals equation to answer the questions in the table below. What are the units of a? What are the units of b? For ammonia the numerical value of a is 4.169 and the numerical value of b is 0.0371. atm Use the van der Waals equation to calculate the pressure of a sample of ammonia at 195.0 °C with a molar volume of 4.12 L/mol. Round your answer to 3 significant digits. Use the Ideal Gas Law to calculate the pressure of the same sample under the same conditions. Round this answer to 3 significant digits also. atm olo
The van der Waals equation of state was designed (by Dutch physicist Johannes van der Waals) to predict the relationship between pressure p, volume V and temperature T for gases better than the Ideal Gas Law does: p+a (V-nb)=nRT The van der Waals equation of state. R stands for the gas constant and n for moles of gas. The parameters a and b must be determined for each gas from experimental data. Use the van der Waals equation to answer the questions in the table below. What are the units of a? What are the units of b? For ammonia the numerical value of a is 4.169 and the numerical value of b is 0.0371. atm Use the van der Waals equation to calculate the pressure of a sample of ammonia at 195.0 °C with a molar volume of 4.12 L/mol. Round your answer to 3 significant digits. Use the Ideal Gas Law to calculate the pressure of the same sample under the same conditions. Round this answer to 3 significant digits also. atm olo
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter5: The Gaseous State
Section: Chapter Questions
Problem 5.103QP: Calculate the molar volume of ethane at 1.00 atm and 0C and at 10.0 atm and 0C, using the van der...
Related questions
Question
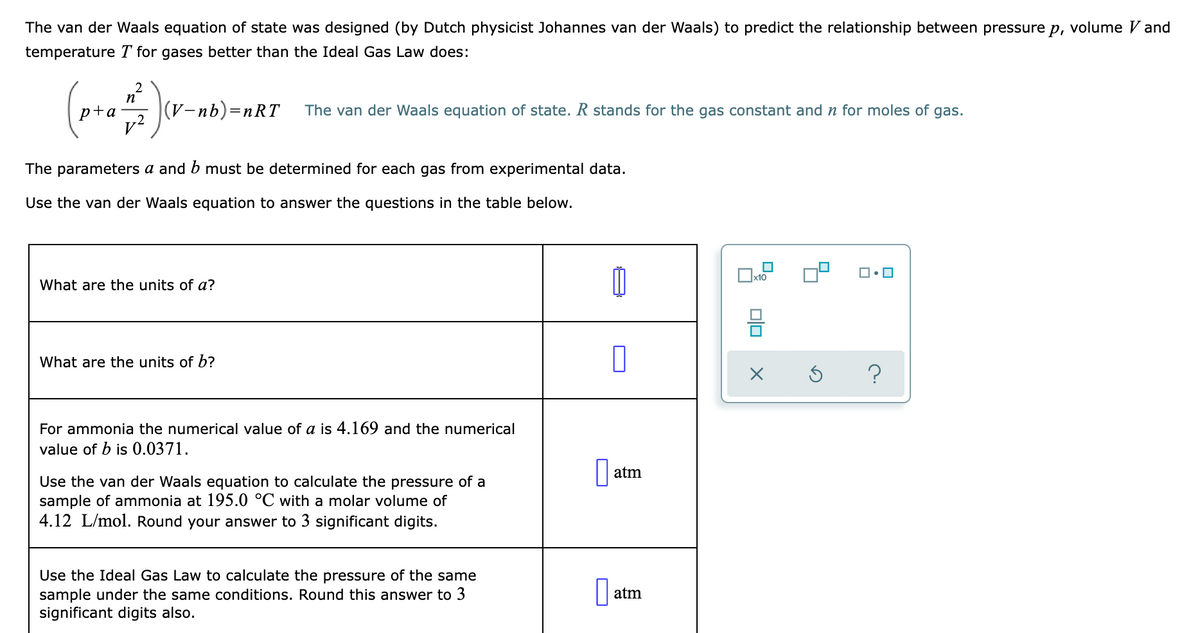
Transcribed Image Text:The van der Waals equation of state was designed (by Dutch physicist Johannes van der Waals) to predict the relationship between pressure p, volume V and
temperature T for gases better than the Ideal Gas Law does:
2
p+a
(V-nb)=nRT
The van der Waals equation of state. R stands for the gas constant and n for moles of gas.
v?
The parameters a and b must be determined for each gas from experimental data.
Use the van der Waals equation to answer the questions in the table below.
What are the units of a?
What are the units of b?
For ammonia the numerical value of a is 4.169 and the numerical
value of b is 0.0371.
atm
Use the van der Waals equation to calculate the pressure of a
sample of ammonia at 195.0 °C with a molar volume of
4.12 L/mol. Round your answer to 3 significant digits.
Use the Ideal Gas Law to calculate the pressure of the same
sample under the same conditions. Round this answer to 3
significant digits also.
|| atm
O
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning
