You (from the graph) Order of the reaction with respect to Nvs (round off to the nearest whole number) Order of the reaction with respect to Luv (round off to the nearest whole number)
Inspired by the sacrifices done by the protector Naevis, Winter was determined to continue Naevis’ experiments with the compounds discovered from Kwangya. Winter searched for all the surviving data from the aftermath of the battle against the Black Mamba and found the following graphs and values.
The general reaction for the formation of compound Ae is as follows:
Nvs + We + Luv + You → Ae
(See picture)
Table 1. Reaction rates of various Ae formation runs at 30 oC.
|
Run |
Concentration of each component in Solution |
Initial rate, M/s |
|||
|
[Nvs], M |
[We], M |
[Luv], M |
[You], M |
||
|
1 |
0.053 |
0.082 |
0.092 |
0.046 |
8.44 x 10-10 |
|
2 |
0.029 |
0.096 |
0.092 |
0.034 |
1.87 x 10-10 |
|
3 |
0.053 |
0.074 |
0.092 |
0.034 |
6.24 x 10-10 |
She also found a numerical value for k = 7.1 x 10-5 but was not able to find its unit. Help her find the following:
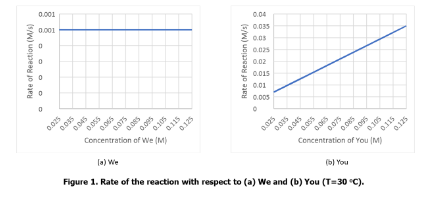
- Order of the reaction with respect to We (from the graph)
- Order of the reaction with respect to You (from the graph)
- Order of the reaction with respect to Nvs (round off to the nearest whole number)
- Order of the reaction with respect to Luv (round off to the nearest whole number)
Step by step
Solved in 2 steps with 2 images









