
Concept explainers
(a)
Interpretation:
It is to be determined if the given pair is not resonance structures of one another.
Concept introduction:
Resonance exists in species for which there are two or more valid Lewis structures. Resonance structures differ only in the placement of their electrons, not their atoms. Resonance stabilization is usually high when the resonance contributors are equivalent. More the number of the resonance contributors, the more is the resonance stabilization.
Answer to Problem 1.75P
The given structures are not resonance structures of one another.
Explanation of Solution
The structures of the given pair are shown below:
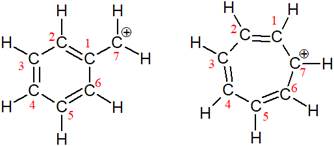
The two structures have completely different carbon skeleton. The first one has a six membered ring while the second has a seven membered ring. Since these two structures do not have the same position of the atoms, they cannot be resonance structures.
From the position of atoms and electrons, it is found that the given pair is not resonance structures of one another.
(b)
Interpretation:
It is to be determined if the given pair is not resonance structures of one another.
Concept introduction:
Resonance exists in species for which there are two or more valid Lewis structures. Resonance structures differ only in the placement of their electrons, not their atoms. Resonance stabilization is usually high when the resonance contributors are equivalent. More the number of the resonance contributors, the more is the resonance stabilization.
Answer to Problem 1.75P
The given structure is not resonance structures of one another.
Explanation of Solution
The structures of the given pair are shown below:
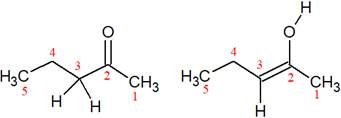
In these two structures, the position of the hydrogen atoms is not the same. Hence, these structures cannot be resonance structures of each other.
From the position of atoms and valence electrons, it is found that the given pair is not resonance structures of one another.
(c)
Interpretation:
It is to be determined if the given pair is not resonance structures of one another.
Concept introduction:
Resonance exists in species for which there are two or more valid Lewis structures. Resonance structures differ only in the placement of their electrons, not their atoms. Resonance stabilization is usually high when the resonance contributors are equivalent. More the number of the resonance contributors, the more is the resonance stabilization.
Answer to Problem 1.75P
The given structure is resonance structures of one another.
Explanation of Solution
The structures of the given pair are as shown below.
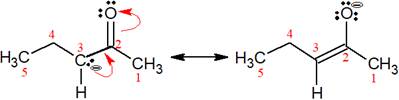
To obtain the second resonance structure, two curved arrows are drawn and the electrons are moved accordingly. The two structures differ only in the placement of their electrons, not their atoms. Therefore, the given pair is resonance structures of one another.
From the position of atoms and valence electrons, it is found that the given pair is resonance structures of one another.
(d)
Interpretation:
It is to be determined if the given pair is not resonance structures of one another.
Concept introduction:
Resonance exists in species for which there are two or more valid Lewis structures. Resonance structures differ only in the placement of their electrons, not their atoms. Resonance stabilization is usually high when the resonance contributors are equivalent. More the number of the resonance contributors, the more is the resonance stabilization.
Answer to Problem 1.75P
The given structure is resonance structures of one another.
Explanation of Solution
The structures of the given pair are shown below:
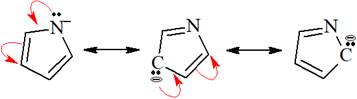
To obtain the second resonance structure, two curved arrows are drawn and the valence electrons are moved accordingly. Similarly, to arrive at the third resonance structure, two curved arrows are drawn and valence electrons are moved accordingly. The resonance structures differ only in the placement of their electrons, not their atoms. Therefore, the given pair is resonance structures of one another.
From the position of atoms and valence electrons, it is found that the given pair is resonance structures of one another.
(e)
Interpretation:
It is to be determined if the given pair is not resonance structures of one another.
Concept introduction:
Resonance exists in species for which there are two or more valid Lewis structures. Resonance structures differ only in the placement of their electrons, not their atoms. Resonance stabilization is usually high when the resonance contributors are equivalent. More the number of the resonance contributors, the more is the resonance stabilization.
Answer to Problem 1.75P
The given structure is resonance structures of one another.
Explanation of Solution
The structures of the given pair are shown below:
![]()
To obtain the second resonance structure, a curved arrow is drawn and the valence electrons are moved accordingly. Similarly, to arrive at the third resonance structure, two curved arrows are drawn and valence electrons are moved accordingly. The resonance structures differ only in the placement of their electrons, not their atoms. Therefore, the given pair is resonance structures of one another.
From the position of atoms and valence electrons, it is found that the given pair is resonance structures of one another.
(f)
Interpretation:
It is to be determined if the given pair is not resonance structures of one another.
Concept introduction:
Resonance exists in species for which there are two or more valid Lewis structures. Resonance structures differ only in the placement of their electrons, not their atoms. Resonance stabilization is usually high when the resonance contributors are equivalent. More the number of the resonance contributors, the more is the resonance stabilization.
Answer to Problem 1.75P
The given structure is not resonance structures of one another.
Explanation of Solution
The structure of the given pair is as follows:
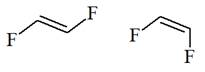
Resonance structures differ only in the placement of their electrons, not their atoms. In the given pair, the placement of electrons is the same. Therefore, the given pair is not resonance structures of one another.
From the position of atoms and valence electrons, it is found that the given pair is not resonance structures of one another.
(g)
Interpretation:
It is to be determined if the given pair is not resonance structures of one another.
Concept introduction:
Resonance exists in species for which there are two or more valid Lewis structures. Resonance structures differ only in the placement of their electrons, not their atoms. Resonance stabilization is usually high when the resonance contributors are equivalent. More the number of the resonance contributors, the more is the resonance stabilization.
Answer to Problem 1.75P
The given structure is not resonance structures of one another.
Explanation of Solution
The structure of the given pair is as follows:
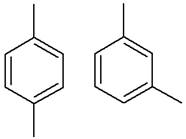
Resonance structures differ only in the placement of their electrons, not their atoms. In the given pair, the placement of electrons is the same. Therefore, the given pair is not resonance structures of one another.
From the position of atoms and valence electrons, it is found that the given pair is resonance structures of one another.
(h)
Interpretation:
It is to be determined if the given pair is not resonance structures of one another.
Concept introduction:
Resonance exists in species for which there are two or more valid Lewis structures. Resonance structures differ only in the placement of their electrons, not their atoms. Resonance stabilization is usually high when the resonance contributors are equivalent. More the number of the resonance contributors, the more is the resonance stabilization.
Answer to Problem 1.75P
The given structure is not resonance structures of one another.
Explanation of Solution
The structure of the given pair is as follows:
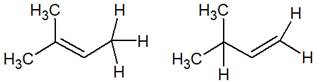
Resonance structures differ only in the placement of their electrons, not their atoms. In the given pair, the placement of electrons is different; at the same time, one H atom changes its position. Therefore, the given pair is not resonance structures of one another.
From the position of atoms and valence electrons, it is found that the given pair is not resonance structures of one another.
Want to see more full solutions like this?
Chapter 1 Solutions
ORG.CHEM W/TEXT+SOLU.MANUAL
- Which of the following resonance structures is incorrectly drawn? a) A b) B c) C d) D e) both A & Darrow_forwardFor each of the following structures,1. Draw a Lewis structure; fill in any nonbonding electrons.2. Calculate the formal charge on each atom other than hydrogen.(a) CH3NO(nitromethane)(b) (CH3)3NO(trimethylamine oxide)(c) [N3]-(azide ion)(d) [(CH3)3O]+ (e) CH3NC (f) (CH3)4NBrarrow_forwardFind all molecules/ions with resonance structures and draw them. HCN CH3OH SeF6 NO2- AsF5 XeF2 TeF5- H2CO SF4 XeF4 PO43- BrF3 NH3 CH3NH2arrow_forward
- Draw out the organic shorthand for the molecules below, and then draw another possible resonance structure of the same molecule next to with arrows to show electron movement. -CH3CH2CONH3 -CH3CH2COOHarrow_forwardWhich of the following resonance structure for OCN- will contribute most to the correct structure of OCN-? O = C-N, with one lone pair on O and three lone pairs on N O=C=N, with two lone pairs on O, and two lone pairs on N O=C=N, with one lone pair on O, two lone pairs on C, and one lone pair on N O-C = N, with three lone pairs on O and one lone pair on N They All contribute equally to the structure on OCN-arrow_forwardWhy is this structure wrong and how can it be corrected? It should be neutral and the formal charge for each atom should be 0.arrow_forward
- Draw the BEST lewis structure (with formal charges) of the indicated chemical species. (Draw all possible resonance structures). 1. HBrO2 2. BrO3^-arrow_forwardAmong the four structures in the choices, one is not a valid resonance form. Identify the wrong structure.arrow_forwardOne resonance structure for O2Cl– ion is drawn below. What is the formal charge on each atom? a. Cl atom = 0 and each O atom = 0 b. Cl atom = 0 and each O atom = –1 c. Cl atom = –1 and each O atom = 0 d. Cl atom = +1 and each O atom = –1 e. Cl atom = 0, one O atom = 0, one O atom = –1arrow_forward
- Show the delocalization of charges in the following structures. Draw the resonance forms and indicate the movement of electrons with curved arrows.Hint: First draw the Lewis structure for each the compound.(i) CH2=CH─O-(ii) CH2=CH─O+arrow_forwardDraw all of the resonance structures for a) NO3- b) NO2- c) HCO3- d) O3arrow_forwardComplete the three Lewis structures for N(NO2)2– by adding missing lone pair electrons and assigning formal charges. Do not add resonance arrows.arrow_forward
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning


