
Concept explainers
a.
Interpretation: The hybridization state and geometry of the following structure need to be predicted:
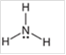
Concept Introduction: The arrangement of electron pairs is identified according to VSEPR (Valence Shell Electron Pair Repulsion) theory wherein all the electron pairs are aligned in such a way that they are at maximum distance from each other. The structure of every molecule in VSEPR is explained by considering the steric number (combination of lone pairs and
b.
Interpretation: The hybridization state and geometry of the following structure need to be predicted:
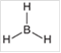
Concept Introduction: The arrangement of electron pairs is identified according to VSEPR (Valence Shell Electron Pair Repulsion) theory wherein all the electron pairs are aligned in such a way that they are at maximum distance from each other. The structure of every molecule in VSEPR is explained by considering the steric number (combination of lone pairs and
c.
Interpretation: The hybridization state and geometry of the following structure need to be predicted:
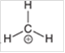
Concept Introduction: The arrangement of electron pairs is identified according to VSEPR (Valence Shell Electron Pair Repulsion) theory wherein all the electron pairs are aligned in such a way that they are at maximum distance from each other. The structure of every molecule in VSEPR is explained by considering the steric number (combination of lone pairs and
d.
Interpretation: The hybridization state and geometry of the following structure need to be predicted:
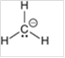
Concept Introduction: The arrangement of electron pairs is identified according to VSEPR (Valence Shell Electron Pair Repulsion) theory wherein all the electron pairs are aligned in such a way that they are at maximum distance from each other. The structure of every molecule in VSEPR is explained by considering the steric number (combination of lone pairs and
Want to see the full answer?
Check out a sample textbook solution
Chapter 1 Solutions
ORGANIC CHEMISTRY,SOLN.MAN.+...-ACCESS
- Draw the Lewis dot diagram, determine the hybridization of interior atoms, draw the molecular geometry and determine the overall polarity of: a. CH3CH₂OH b. CH3COOH C. CH3COOCH3arrow_forwardWhat is the molecular geometry for the carbon atom in COCI,? Select one: a. linear b. tetrahedral c. trigonal pyramidal d. trigonal planar e. bentarrow_forwardWhich of the following is the wedge and dash structure for the following molecule? Et A B C D H Et H A. B. C. D.arrow_forward
- Give detailed Solution with explanation neededarrow_forwardTwo important industrial chemicals, ethene, C2H4, and propene, C3H6, are produced by the steam (or thermal) cracking process: 2C3H8(g)C2H4(g)+C3H6(g)+H2(g) For each of the four carbon compounds, do the following: (a) Draw a Lewis structure. (b) Predict the geometry about the carbon atom. (c) Determine the hybridization of each type of carbon atom.arrow_forward• predict the geometry of a molecule from its, Lewis structure.arrow_forward
- Which model best describes why bonds form between some atoms, but not others? A. Valence bond diagram b. Molecular orbital diagram c. Potential energy diagramarrow_forwardWhich of the following can be best explained by temporary dipole moments? Choose one or more: A. This explains why ammonia and nitrogen gas exhibit an attractive force between them. B. This explains how the molecules hydrogen fluoride and methanol can exhibit uncharacteristically strong intermolecular forces. C. This explains why long hydrocarbon chains have relatively high boiling points. D. This explains how two noble gases' molecules can have an attractive force between them. E. This explains why the dipole-dipole attractive force between dimethyl ether and acetone does not entirely account for the attractive force between these molecules.arrow_forwardfor each of the following compounds... a. Provide the missing name line structure b. Build a model using the molecular model kit c. Draw the lewis structure of the model and indicate the hybridization of each ( atom. orarrow_forward
- B. Write the molecular geometry of the following three structures: II III Accessibility. Investigate D. Focusarrow_forwardIn a tetrahedral geometry resulted from orbital hybridization, how many unhybridized atomic orbitals remain? Question 2 options: a. One p orbital b. Three d orbitals c. None d. Two p orbitals e. Four d orbitalsarrow_forwardCF has a Lewis structure with four the bond angles are bonds and degrees. The molecule is lone pairs. The 3-D geometry of the molecule is and is therefore insoluble in water. andarrow_forward
 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning





